Question
A (1.50x10^1) liter bottle is filled with nitrogen (N2) at STP and closed tight. (STP means Standard Temperature and Pressure, i.e. 273 K and
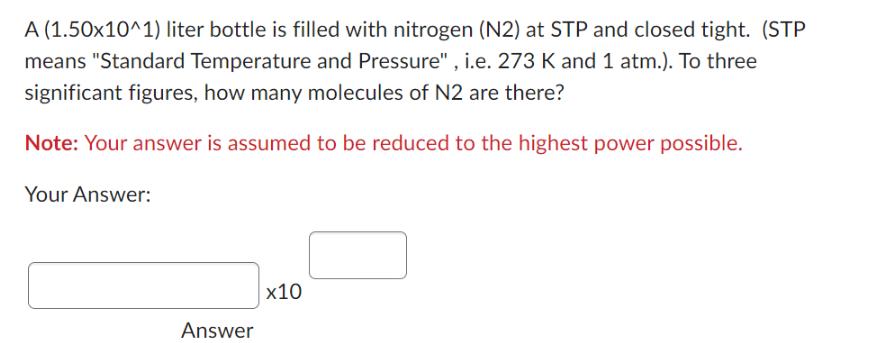
A (1.50x10^1) liter bottle is filled with nitrogen (N2) at STP and closed tight. (STP means "Standard Temperature and Pressure", i.e. 273 K and 1 atm.). To three significant figures, how many molecules of N2 are there? Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: Answer x10
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
A nice problem First lets find the number of moles of N2 in the bottle mo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Thermodynamics
Authors: Richard E. Sonntag, Claus Borgnakke, Gordon J. Van Wylen
6th edition
471152323, 978-0471152323
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



