Answered step by step
Verified Expert Solution
Question
1 Approved Answer
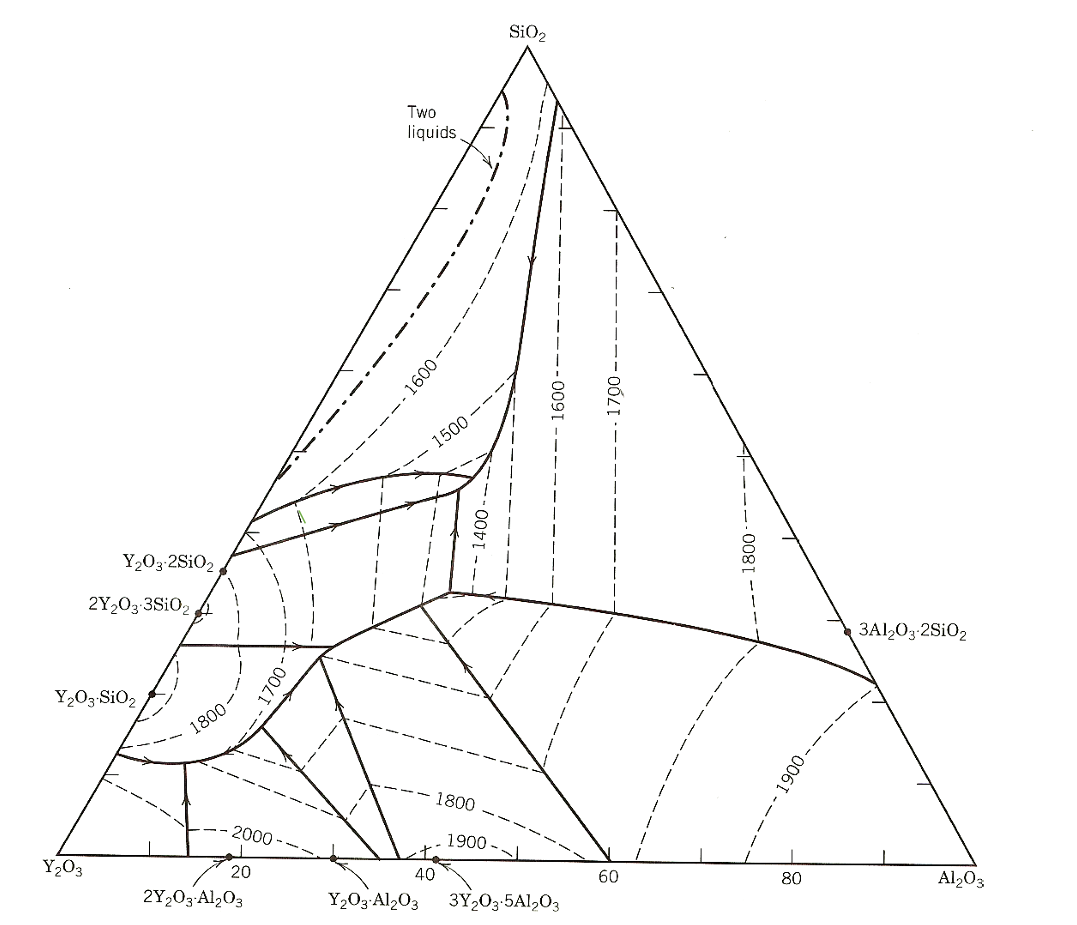
( a ) A small amount of SiO 2 powder ( 5 wt % ) is added to a fine YAG powder, in hopes of
a A small amount of SiO powder wt is added to a fine YAG powder, in hopes of densifying the powder compact into a transparent polycrystaline YAG suitable for IR window material by forming a minor amount of liquid phase during firing. After mixing uniformly, the powder mixture is pressed into a pellet and rapidly heated in a furnace. What is the minimum temperature approximately at which a liquid phase might form? What is the corresponding liquid composition?
b After firing the pellet at deg C for many hours, the sample is cooled to room temperature at a rate slow enough to maintain equilibrium. Upon reheating, at what temperature would a liquid first form in this sample? What would be the liquid composition? Explain how you arrived at your answer.
c In a separate study, fine YO and SiO powder are added to AlO powder as liquidphase sintering aids. The overall composition is wt AlO SiO and YO If the sample is equilibrated at deg C what phases are present, and in what amounts?
d Toward the SiOrich end of the SiOYO join, there exists a region of liquid immiscibility where two liquid phases are present at equilibrium. Can you determine from this phase diagram the exact compositions of the two liquids? The approximate composition? Explain.
e What is the most refractory highest melting threephase composite one can prepare in this ternary system?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


