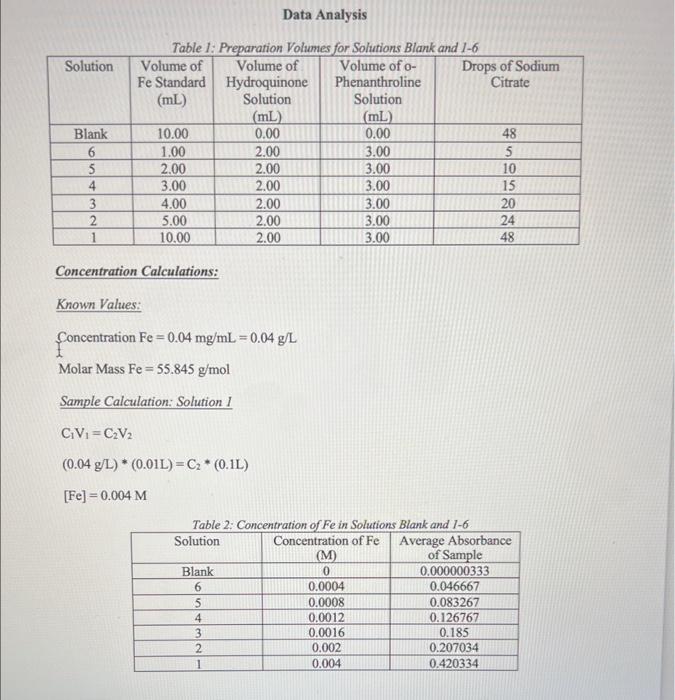
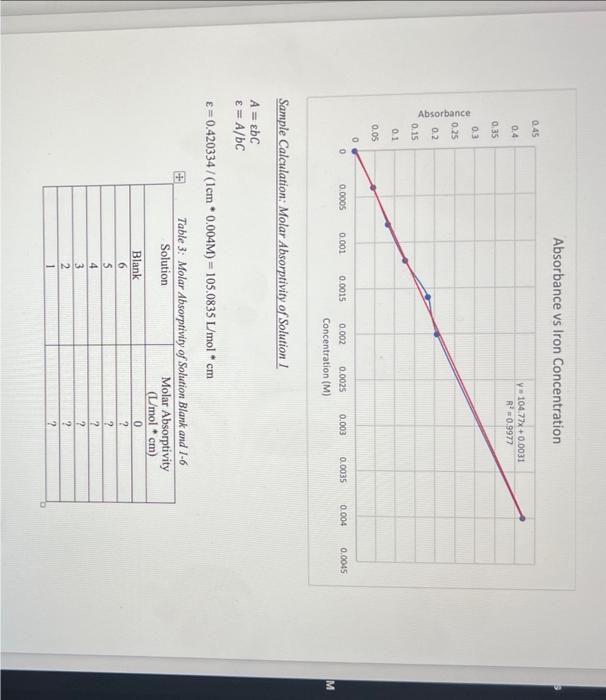
a) calculate the molar absorptivity of solutions 1-6 and the blank using Beers Law assuming the cuvets path length is 1cm
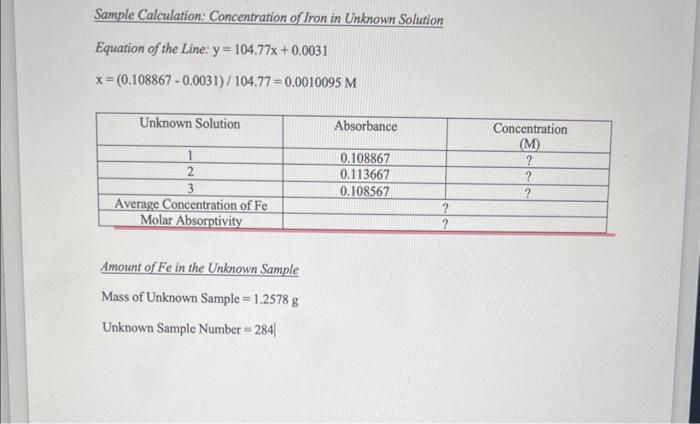
b) calculate the molarity (concentration) of Fe (o-phenanthroline) in the unknown sample (rememebr that all the iron has been converted to the the phenanthroline complex)
c) determine the number of milligrams of Fe in the unknown sample.
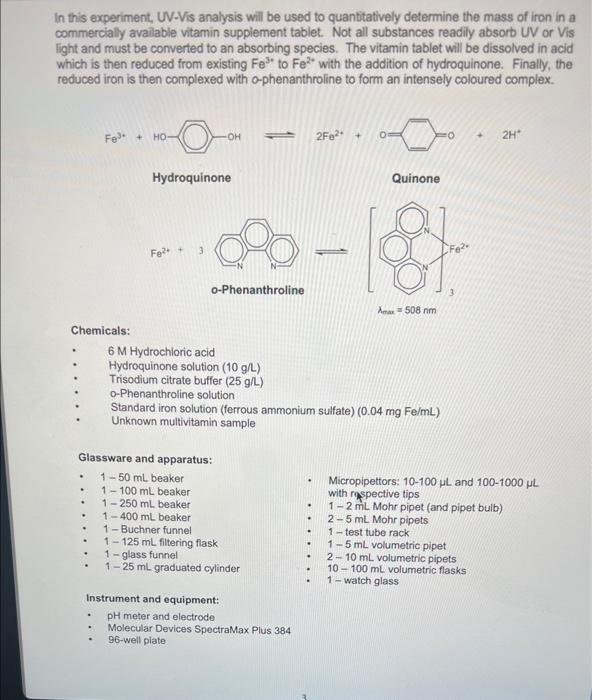
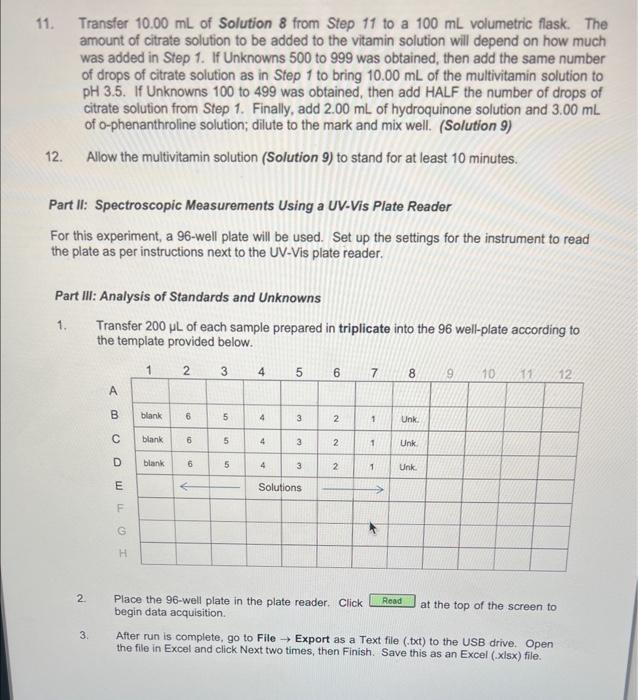
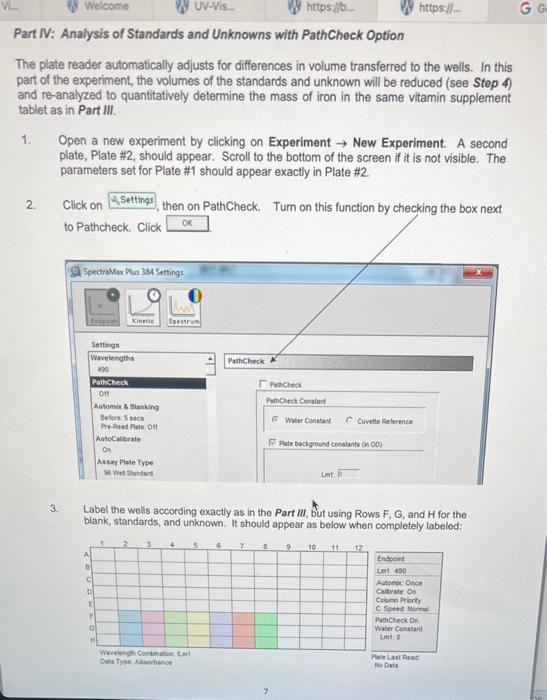
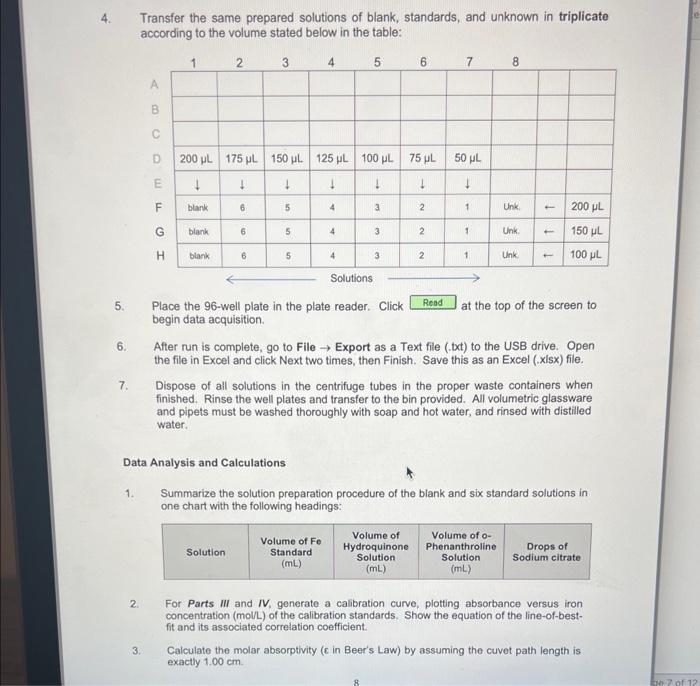
In this experiment, UV-Vis analysis will be used to quantitatively determine the mass of iron in a commercially available vitamin supplement tablet. Not all substances readily absorb UV or Vis light and must be converted to an absorbing species. The vitamin tablet will be dissolved in acid which is then reduced from existing Fe3 to Fe2 with the addition of hydroquinone. Finally, the reduced iron is then complexed with o-phenanthroline to form an intensely coloured complex. 2Fe2+ Hydroquinone Quinone o-Phenanthroline max=508nm Chemicals: - 6M Hydrochloric acid - Hydroquinone solution ( 10g/L) - Trisodium citrate buffer (25g/L) - o-Phenanthroline solution - Standard iron solution (ferrous ammonium sulfate) (0.04mgFe/mL) Unknown multivitamin sample Glassware and apparatus: - 1-50 mL beaker - 1100mL beaker - Micropipettors: 10100L and 1001000L - 1250mL beaker with rispective tips - 1400mL beaker - 12mL Mohr pipet (and pipet bulb) - 1 - Buchner funnel - 25mL Mohr pipets - 1125mL filtering flask - 1 -test tube rack - 1 - glass funnel - 15mL volumetric pipet - 125mL graduated cylinder - 210mL volumetric pipets - 10100mL volumetric flasks - 1 -watch glass Instrument and equipment: - pH meter and electrode - Molecular Devices SpectraMax Plus 384 - 96-well plate Part I: Preparation of Unknown Multivitamin and Standard Solutions Record all measurements and the sample identification number of the unknown multivitamin. Standard Preparation 1. Accurately pipet 10.00mL of standard Fe solution with a volumetric pipet into a 50mL beaker and measure the pH with a glass electrode. Add sodium citrate solution one drop at a time using a disposable pipet until a pH of 3.5 is reached. Count and record the drops needed (approximately 40-60 drops). 2. Pipet a fresh 10.00mL aliquot of Fe standard into a 100mL volumetric flask and add the same number of drops of citrate solution as required in Step 1. Add 2.00mL of hydroquinone solution and 3.00mL of o-phenanthroline solution, dilute to the mark with deionized water, and mix well. (Solution 1) 3. Prepare additional solutions from 5.00,4.00,3.00,2.00, and 1.00mL of Fe standard. Add 2.00mL of hydroquinone solution and 3.00mL of o-phenanthroline solution to each standard solution. Use sodium citrate solution in proportion to the volume of Fe solution. (If 10mL of Fe requires 40 drops of citrate solution, 5mL of Fe requires 20 drops of citrate solution.) Dilute to the mark with deionized water, and mix well. (Solutions 2 to 6 and Blank) 4. Allow all solutions to stand for at least 10 minutes before taking any spectrophotometric measurements. Vitamin Preparation 5. Obtain an unknown multivitamin sample from the GA and mass 1.2500g(0.01g) into a 100mL beaker. Add 25mL of 6MHCl to the beaker. Cover with a watch glass and boil gently for 15 minutes. Figure 1.3. Vacuum filtration apparatus using a Buchner funnel. 7. Turn on the vacuum and swirl the solution before adding it to the Buchner funnel. Try to remove any particles still adhering to the walls of the beaker with deionized water and transfer the washings to the Buchner funnel. Note: Monitor the volume when washing and fittering with deionized water, ensuring less than 50mL of deionized water is used. 8. Break the vacuum seal before turning off the vacuum to prevent backflow. Turn off the vacuum once the precipitate has been sucked reasonably dry. The filter paper may be disposed in the regular garbage container. 9. Obtain a 100mL volumetric flask and glass funnel, and transfer the filtered solution to the volumetric flask (Solution 7). Rinse the filtering flask thoroughly with deionized water into the volumetric flask to quantitatively transfer all of the vitamin solution. Allow the solution to cool to room temperature before completing the volume with deionized water to the interval mark. Invert the flask several then to ensure complete mixing. Note: It is important that the solution cools to room temperature, since volumetric flask marks are accurate only when at room temperature. 10. Accurately transfer a volume of this multivitamin solution (with a calibrated volumetric pipet) to a clean 100mL volumetric flask according to the unknown below. Complete the volume with deionized water. (Solution 8) 11. Transfer 10.00mL of Solution 8 from Step 11 to a 100mL volumetric flask. The amount of citrate solution to be added to the vitamin solution will depend on how much was added in Step 1. If Unknowns 500 to 999 was obtained, then add the same number of drops of citrate solution as in Step 1 to bring 10.00mL of the multivitamin solution to pH3.5. If Unknowns 100 to 499 was obtained, then add HALF the number of drops of citrate solution from Step 1. Finally, add 2.00mL of hydroquinone solution and 3.00mL of o-phenanthroline solution; dilute to the mark and mix well. (Solution 9) 12. Allow the multivitamin solution (Solution 9) to stand for at least 10 minutes. Part II: Spectroscopic Measurements Using a UV-Vis Plate Reader For this experiment, a 96 -well plate will be used. Set up the settings for the instrument to read the plate as per instructions next to the UV-Vis plate reader. Part III: Analysis of Standards and Unknowns 1. Transfer 200L of each sample prepared in triplicate into the 96 well-plate according to the template provided below. 2. Place the 96-well plate in the plate reader. Click begin data acquisition. at the top of the screen to 3. After run is complete, go to File Export as a Text file (.txt) to the USB drive. Open the file in Excel and click Next two times, then Finish. Save this as an Excel (xisx) file. Part IV: Analysis of Standards and Unknowns with PathCheck Option The plate reader automatically adjusts for differences in volume transferred to the wells. In this part of the experiment, the volumes of the standards and unknown will be reduced (see Step 4) and re-analyzed to quantitatively determine the mass of iron in the same vitamin supplement tablet as in Part III. 1. Open a new experiment by clicking on Experiment New Experiment. A second plate, Plate #2, should appear. Scroll to the bottom of the screen if it is not visible. The parameters set for Plate \#1 should appear exactly in Plate \#2. 2. Click on then on PathCheck. Turn on this function by checking the box next to Pathcheck. Click 3. Label the wells according exactly as in the Part III, but using Rows F,G, and H for the blank, standards, and unknown. It should appear as below when completely labeled: Transfer the same prepared solutions of blank, standards, and unknown in triplicate according to the volume stated below in the table: 5. Place the 96-well plate in the plate reader. Click at the top of the screen to begin data acquisition. 6. After run is complete, go to File Export as a Text file (.txt) to the USB drive. Open the file in Excel and click Next two times, then Finish. Save this as an Excel (.xlsx) file. 7. Dispose of all solutions in the centrifuge tubes in the proper waste containers when finished. Rinse the well plates and transfer to the bin provided. All volumetric glassware and pipets must be washed thoroughly with soap and hot water, and rinsed with distilled water. Data Analysis and Calculations 1. Summarize the solution preparation procedure of the blank and six standard solutions in one chart with the following headings: 2. For Parts III and IV, generate a calibration curve, plotting absorbance versus iron concentration (moll) of the calibration standards. Show the equation of the line-of-bestfit and its associated correlation coefficient. 3. Calculate the molar absorptivity ( in Beer's Law) by assuming the cuvet path length is exactly 1.00cm. Data Analysis Concentration Calculations: Known Values: Concentration Fe=0.04mg/mL=0.04g/L Molar Mass Fe=55.845g/mol Sample Calculation: Solution I C1V1=C2V2(0.04g/L)(0.01L)=C2(0.1L)[Fe]=0.004M Tahle 9. Cancontration of Fo in. Solutione Rlank and 1-6 Sample Calculation: Molar Absorptivity of Solution I A=bC=A/bC =0.420334/(1cm0.004M)=105.0835L/molcm Sample Calculation: Concentration of Iron in Unknown Solution Equation of the Line: y=104.77x+0.0031 x=(0.1088670.0031)/104.77=0.0010095M Amotent of Fe in the Unknown Sample Mass of Unknown Sample =1.2578g Unknown Sample Number =284