Question
A CO stream is available at 1 barA and 25C and is fully saturated with water. It is to be compressed up to 150
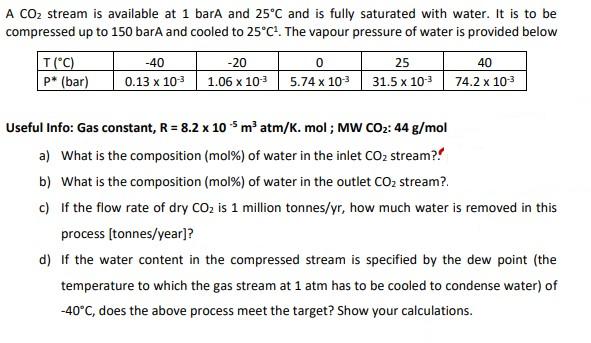
A CO stream is available at 1 barA and 25C and is fully saturated with water. It is to be compressed up to 150 barA and cooled to 25C. The vapour pressure of water is provided below T (C) p* (bar) -40 0.13 x 10 -20 1.06 x 10- 0 25 5.74 x 10- 31.5 x 10- 40 74.2 x 10- Useful Info: Gas constant, R = 8.2 x 105 m atm/K. mol; MW CO: 44 g/mol a) What is the composition (mol %) of water in the inlet CO stream?? b) What is the composition (mol %) of water in the outlet CO stream?. c) If the flow rate of dry CO is 1 million tonnes/yr, how much water is removed in this process [tonnes/year]? d) If the water content in the compressed stream is specified by the dew point (the temperature to which the gas stream at 1 atm has to be cooled to condense water) of -40C, does the above process meet the target? Show your calculations.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



