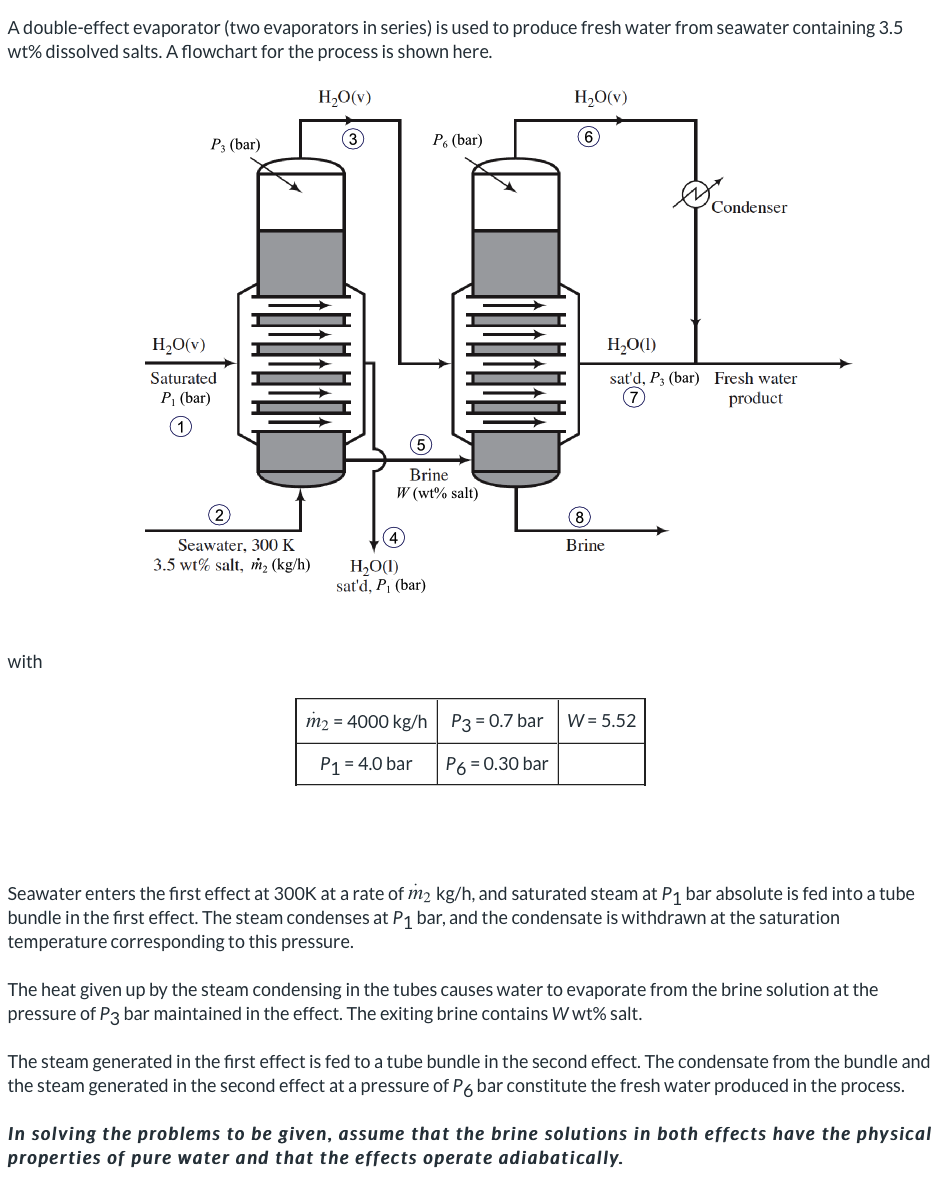
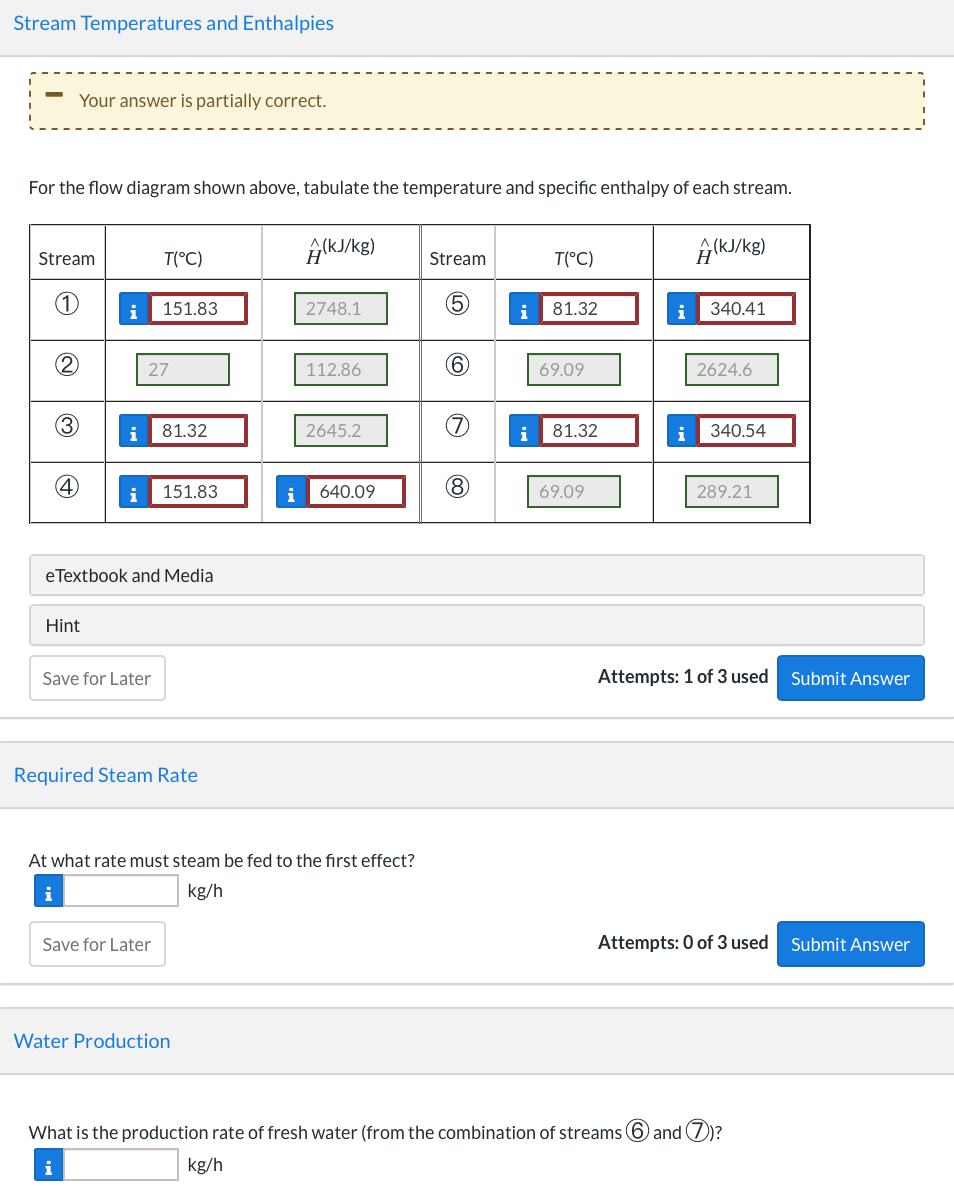
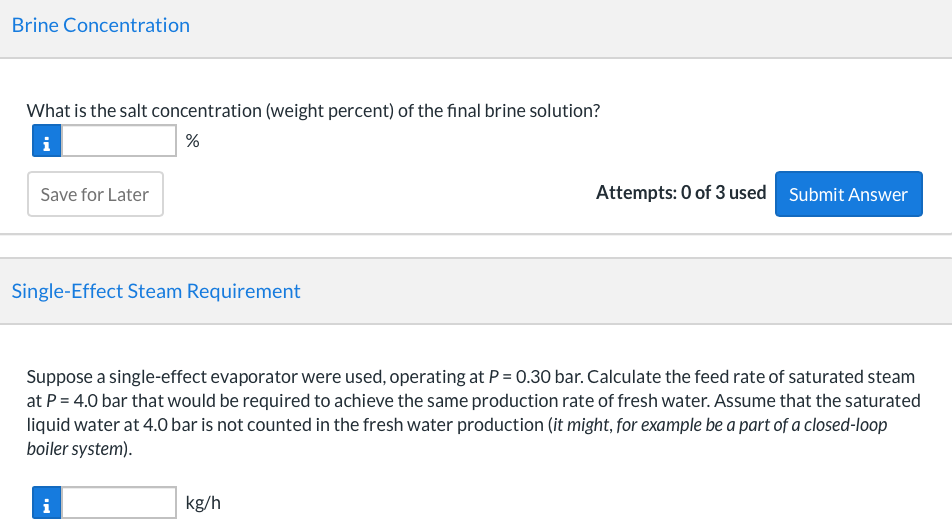
A double-effect evaporator (two evaporators in series) is used to produce fresh water from seawater containing 3.5 wt% dissolved salts. A flowchart for the process is shown here. H2O(v) H2O(V) P3 (bar) P. (bar) Condenser H2O(v) Saturated P (bar) H2O(1) sat'd, P3 (bar) Fresh water product Brine W (wt% salt) Brine Seawater, 300 K 3.5 wt% salt, m2 (kg/h) HO(1) sat'd, P. (bar) with m2 = 4000 kg/h P3 = 0.7 bar W=5.52 P1 = 4.0 bar P6 = 0.30 bar Seawater enters the first effect at 300K at a rate of m2 kg/h, and saturated steam at P1 bar absolute is fed into a tube bundle in the first effect. The steam condenses at P1 bar, and the condensate is withdrawn at the saturation temperature corresponding to this pressure. The heat given up by the steam condensing in the tubes causes water to evaporate from the brine solution at the pressure of P3 bar maintained in the effect. The exiting brine contains W wt% salt. The steam generated in the first effect is fed to a tube bundle in the second effect. The condensate from the bundle and the steam generated in the second effect at a pressure of P6 bar constitute the fresh water produced in the process. In solving the problems to be given, assume that the brine solutions in both effects have the physical properties of pure water and that the effects operate adiabatically. Stream Temperatures and Enthalpies Your answer is partially correct. For the flow diagram shown above, tabulate the temperature and specific enthalpy of each stream. Stream T(C) (kJ/kg) Stream T(C) (kJ/kg) 1 i 151.83 2748.1 5 81.32 i 340.41 2 27 112.86 6 69.09 2624.6 3 i 81.32 2645.2 i 81.32 i 340.54 4 i 151.83 8 640.09 i 69.09 289.21 e Textbook and Media Hint Save for Later Attempts: 1 of 3 used Submit Answer Required Steam Rate At what rate must steam be fed to the first effect? i kg/h Save for Later Attempts: 0 of 3 used Submit Answer Water Production What is the production rate of fresh water (from the combination of streams and ? kg/h i Brine Concentration What is the salt concentration (weight percent) of the final brine solution? i % Save for Later Attempts: 0 of 3 used Submit Answer Single-Effect Steam Requirement Suppose a single-effect evaporator were used, operating at P = 0.30 bar. Calculate the feed rate of saturated steam at P = 4.0 bar that would be required to achieve the same production rate of fresh water. Assume that the saturated liquid water at 4.0 bar is not counted in the fresh water production (it might, for example be a part of a closed-loop boiler system). i kg/h









