Question
a. For a 30% mixture that starts out subcooled and then is heated, at what temperature will begin to form vapor (bubbles)? What is

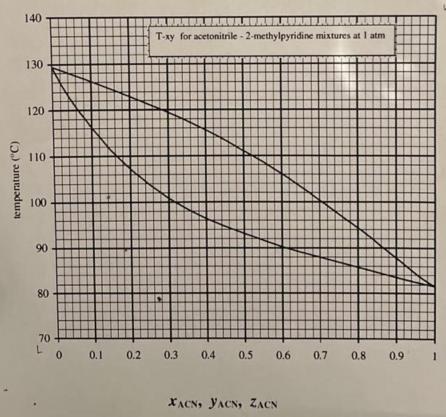
a. For a 30% mixture that starts out subcooled and then is heated, at what temperature will begin to form vapor (bubbles)? What is the composition of the first bubbles formed? b. For a 30% mixture that starts out superheated and then is cooled, at what temperature will begin to form liquid (dew drops)? What is the composition of the first condensate formed? c. For a 30% mixture at 225F, what fraction of the mixture is vapor, and what fraction is in the form of liquid? What are the liquid and vapor phase compositions? d. At what temperature will the vapor fraction be 50% (a 1:1 vapor to liquid ratio)? temperature (C) 140 130 120 110 100 90 80 T-xy for acetonitrile-2-methylpyridine mixtures at I atm L 71 70+ 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 XACN, YACN, ZACN
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Engineering Mechanics Statics & Dynamics
Authors: Russell C. Hibbeler
15th Edition
0134895150, 9780134895154
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



