Answered step by step
Verified Expert Solution
Question
1 Approved Answer
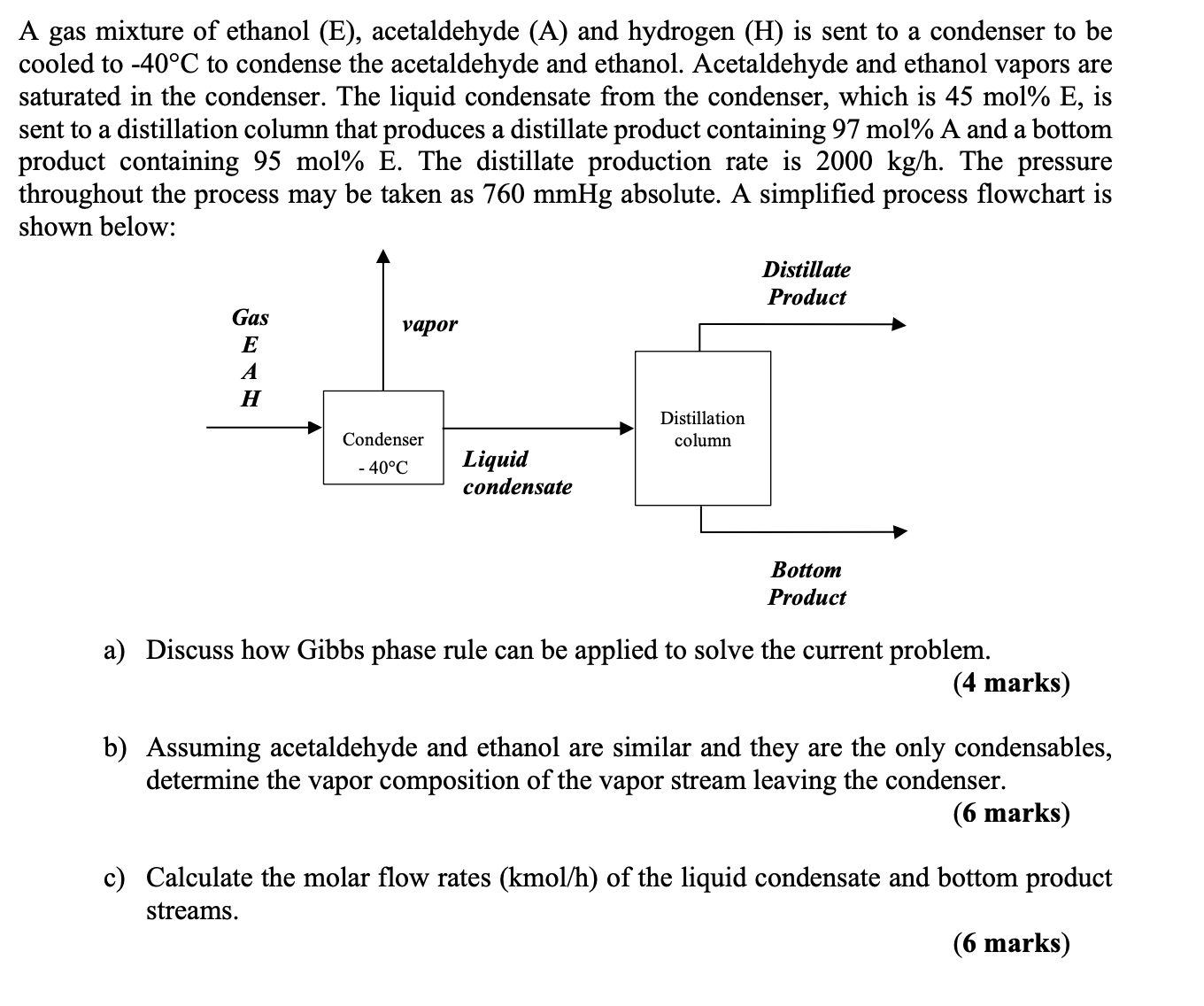
A gas mixture of ethanoA gas mixture of ethanol ( E ) , acetaldehyde ( A ) and hydrogen ( H ) is sent to
A gas mixture of ethanoA gas mixture of ethanol acetaldehyde and hydrogen is sent to a condenser to be
cooled to to condense the acetaldehyde and ethanol. Acetaldehyde and ethanol vapors are
saturated in the condenser. The liquid condensate from the condenser, which is mol is
sent to a distillation column that produces a distillate product containing molA and a bottom
product containing mol The distillate production rate is The pressure
throughout the process may be taken as absolute A simplified process flowchart is
shown below:
Product
a Discuss how Gibbs phase rule can be applied to solve the current problem.
marks
b Assuming acetaldehyde and ethanol are similar and they are the only condensables,
determine the vapor composition of the vapor stream leaving the condenser.
marks
c Calculate the molar flow rates of the liquid condensate and bottom product
streams.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


