Question: A laboratory flotation test on a copper sulphide ore gave the following results: a) Calculate the concentration, Ct, remaining in the 3 litre cell after
A laboratory flotation test on a copper sulphide ore gave the following results:
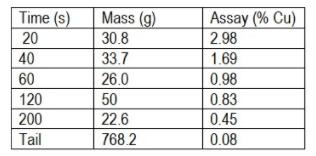
a) Calculate the concentration, Ct, remaining in the 3 litre cell after time t.
b) Determine the rate constant.
c) If a bank of continuous cells has the same rate constant, how many cells would be required to achieve a recovery of 85 % if the cell size is 16 m3 and the feed is 1344 m3/h.
Step by Step Solution
3.40 Rating (159 Votes )
There are 3 Steps involved in it
Mass a Time 298 308 20 16 9 40 337 60 098 260 120 083 200 22... View full answer

Get step-by-step solutions from verified subject matter experts


