Question
A power plant emits a carbon containing pollutant X to the atmosphere at a constant rate E (kg s -1 ). X is removed from
A power plant emits a carbon containing pollutant X to the atmosphere at a constant rate E (kg s-1). X is removed from the atmosphere by a chemical reaction with a first-order rate constant k (s-1), forming the greenhouse gas CO2. X is not a greenhouse gas, and X in the atmosphere has reached a steady state (e.g. constant mole fractions and completely mixed in the entire atmosphere). The emitted pollutant X has a 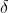 13C value of -20 (VPDB, 13RVPDB=0.011117). The chemical removal of X fractionates with a fractionation factor a= k13/ k = 0.988.
13C value of -20 (VPDB, 13RVPDB=0.011117). The chemical removal of X fractionates with a fractionation factor a= k13/ k = 0.988.
a) Write down the mass balance of the rare isotope in terms of Nc (total number of molecules removed) and Ne (total number of molecules emitted) and the fractionation factor a and the isotope ratio of the emission RE and the isotope ratio of pollutant X in the atmosphere RA. Assume a steady state condition
b) Calculate the 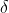 value of the atmosphere from the previously found mass balance.
value of the atmosphere from the previously found mass balance.
c) What is the mass balance of the rare isotope if, in addition to the chemical loss in the atmosphere, the pollutant is also removed by rain, e.g. wet deposition. This last process occurs without fractionation. Consider again a stationary state (in and outflow are equal). The fractional contributions of the chemical loss to the removal is x, and that of wet deposition is y, with x + y = 1.
d) Suppose we observe a 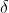 13C value of -15 with respect to VPDB in the atmosphere (in pollutant X), and given the isotopic composition of the emissions, what are then the relative contributions of the two removal mechanisms, e.g. what is x and x-1?
13C value of -15 with respect to VPDB in the atmosphere (in pollutant X), and given the isotopic composition of the emissions, what are then the relative contributions of the two removal mechanisms, e.g. what is x and x-1?
e) If, due to global warming, the amount of rain would increase, would that increase or decrease the global warming impact of pollutant X?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


