Answered step by step
Verified Expert Solution
Question
1 Approved Answer
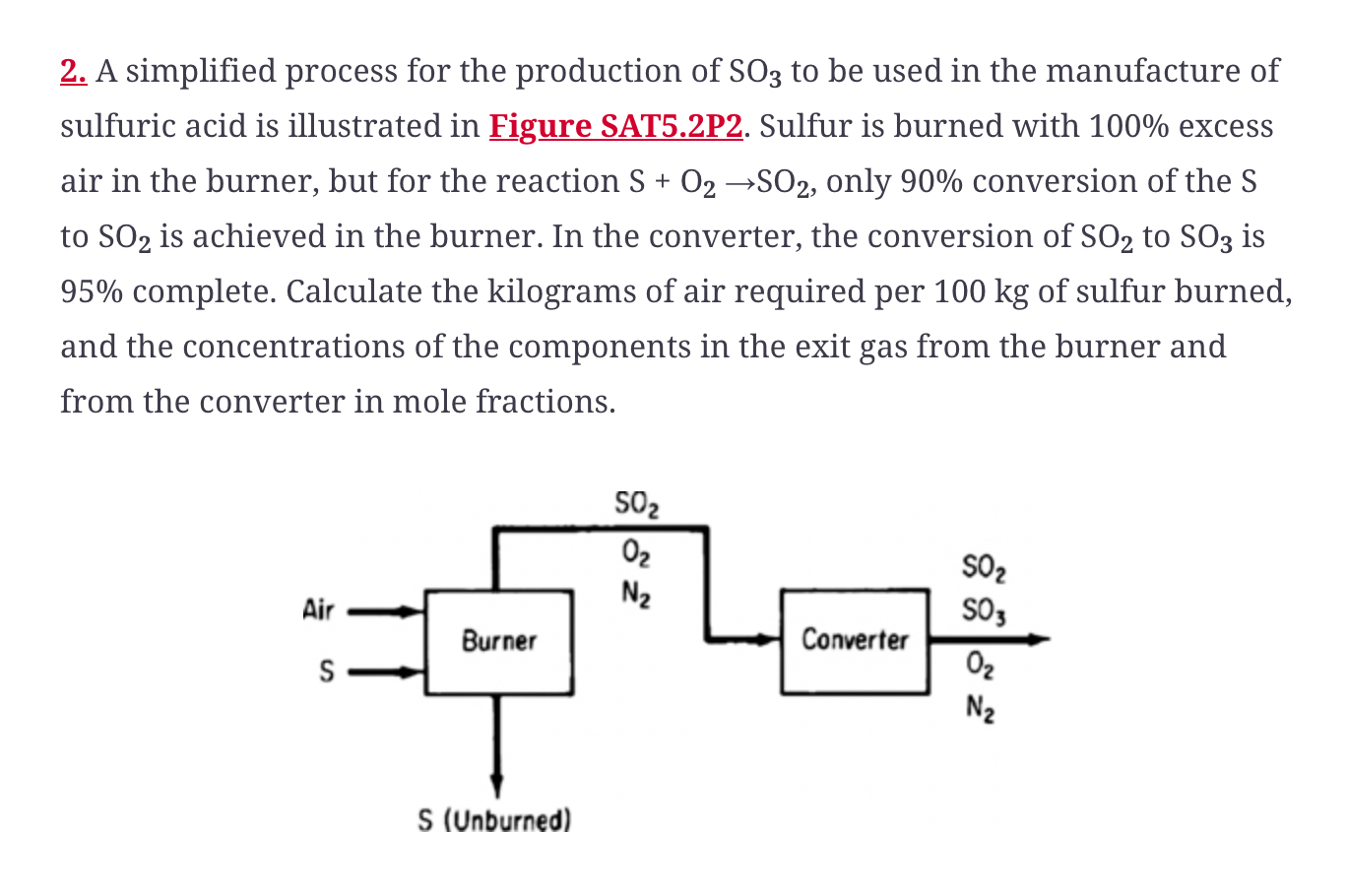
A simplified process for the production of S O 3 to be used in the manufacture of sulfuric acid is illustrated in Figure SAT 5
A simplified process for the production of to be used in the manufacture of sulfuric acid is illustrated in Figure SATP Sulfur is burned with excess air in the burner, but for the reaction only conversion of the to is achieved in the burner. In the converter, the conversion of to is complete. Calculate the kilograms of air required per of sulfur burned, and the concentrations of the components in the exit gas from the burner and from the converter in mole fractions.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


