Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A solution of F-is prepared by dissolving 0.0997 + 0.0004 g NaF (molar mass = 41.989 + 0.001 g/mol) in 162.00 + 0.06 mL of
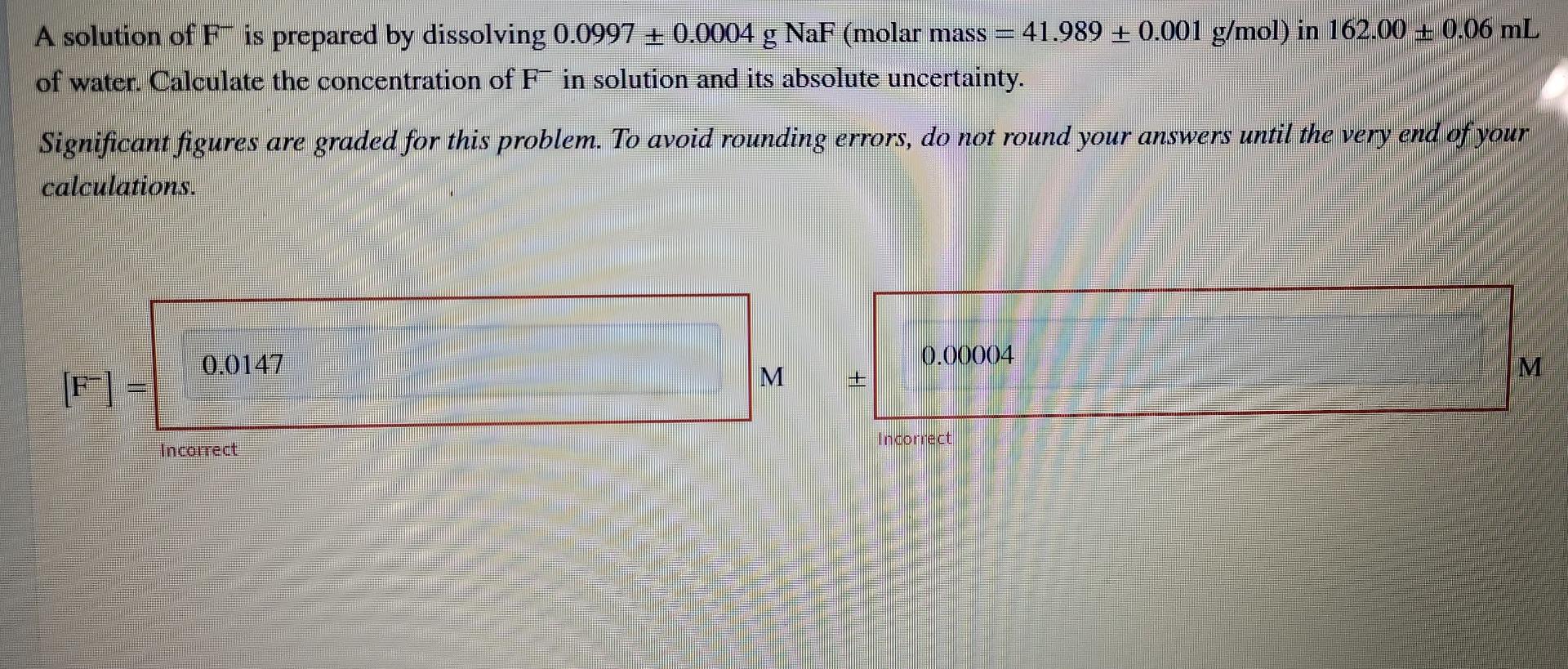
A solution of F-is prepared by dissolving 0.0997 + 0.0004 g NaF (molar mass = 41.989 + 0.001 g/mol) in 162.00 + 0.06 mL of water. Calculate the concentration of F in solution and its absolute uncertainty. end of your Significant figures are graded for this problem. To avoid rounding errors, do not round your answers until the very calculations. 0.0147 0.00004 M M It [F] = - Incorrect Incorrect
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


