Answered step by step
Verified Expert Solution
Question
1 Approved Answer
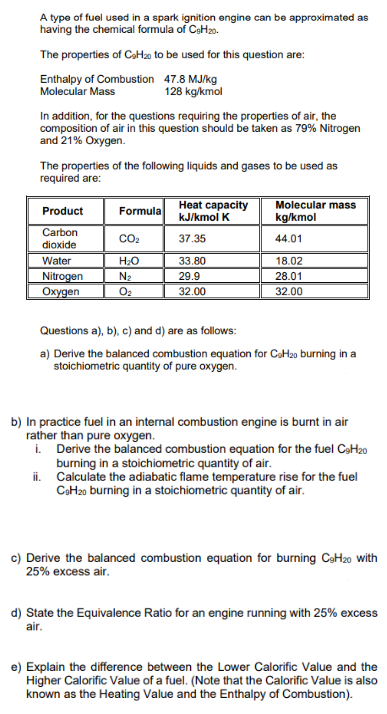
A type of fuel used in a spark ignition engine can be approximated as having the chemical formula of C 6 H 2 0 .
A type of fuel used in a spark ignition engine can be approximated as
having the chemical formula of
The properties of to be used for this question are:
Enthalpy of Combustion
Molecular Mass
mol
In addition, for the questions requiring the properties of air, the
composition of air in this question should be taken as Nitrogen
and Oxygen.
The properties of the following liquids and gases to be used as
required are:
Questions a b c and d are as follows:
a Derive the balanced combustion equation for burning in a
stoichiometric quantity of pure oxygen.
b In practice fuel in an internal combustion engine is burnt in air
rather than pure oxygen.
i Derive the balanced combustion equation for the fuel
burning in a stoichiometric quantity of air.
ii Calculate the adiabatic flame temperature rise for the fuel
burning in a stoichiometric quantity of air.
c Derive the balanced combustion equation for burning with
excess air.
d State the Equivalence Ratio for an engine running with excess
air.
e Explain the difference between the Lower Calorific Value and the
Higher Calorific Value of a fuel. Note that the Calorific Value is also
known as the Heating Value and the Enthalpy of Combustion
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


