Question
Apply the Gibbs phase rule and obtain the number of degree of freedom (F) to the various points (Point 1 to Point 5) in
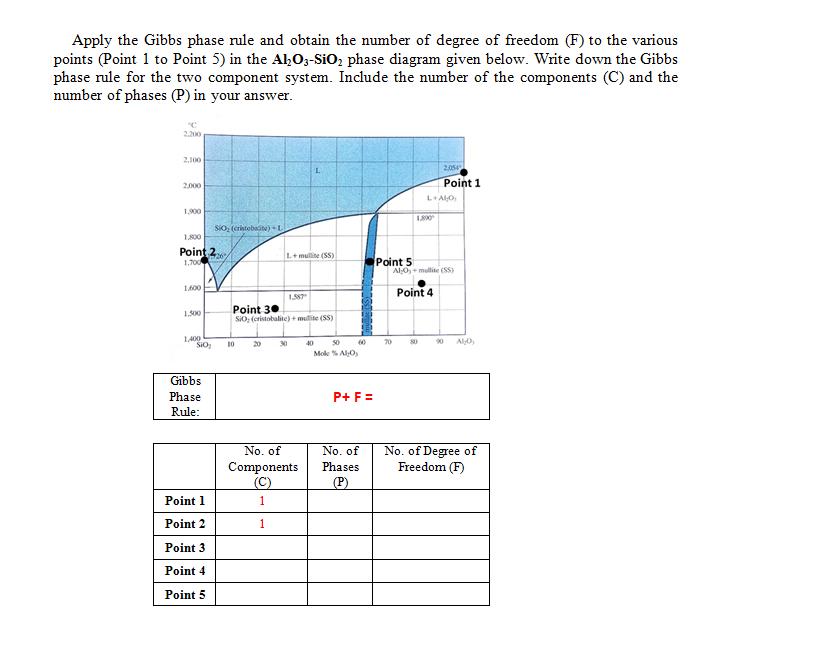
Apply the Gibbs phase rule and obtain the number of degree of freedom (F) to the various points (Point 1 to Point 5) in the AlO3-SiO phase diagram given below. Write down the Gibbs phase rule for the two component system. Include the number of the components (C) and the number of phases (P) in your answer. 2.200 2,100 2,000 1,900 1,800 Point 27 1,700 1,600 1.500 1,400 SIO Gibbs Phase Rule: SiO (cristobacite)+ L Point 1 Point 2 Point 3 Point 4 Point 5 10 L+mullite (SS) Point 30 SiO, (cristobalite) + multe (55) 20 1.587 30 No. of Components (C) 1 1 40 50 Mol % Alo M 60 P+ F = No. of Phases (P) Point 5 70 1.800 L+ALO 80 2054 Point 1 AlO, mullite (SS) Point 4 90 ALO No. of Degree of Freedom (F)
Step by Step Solution
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Materials Science and Engineering An Introduction
Authors: William D. Callister Jr., David G. Rethwisch
8th edition
470419970, 978-0470419977
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



