Question
As shown in the figure above, hexane (C 6 H 14 , H) and pentane (C 5 H 12 , P) are separated in a
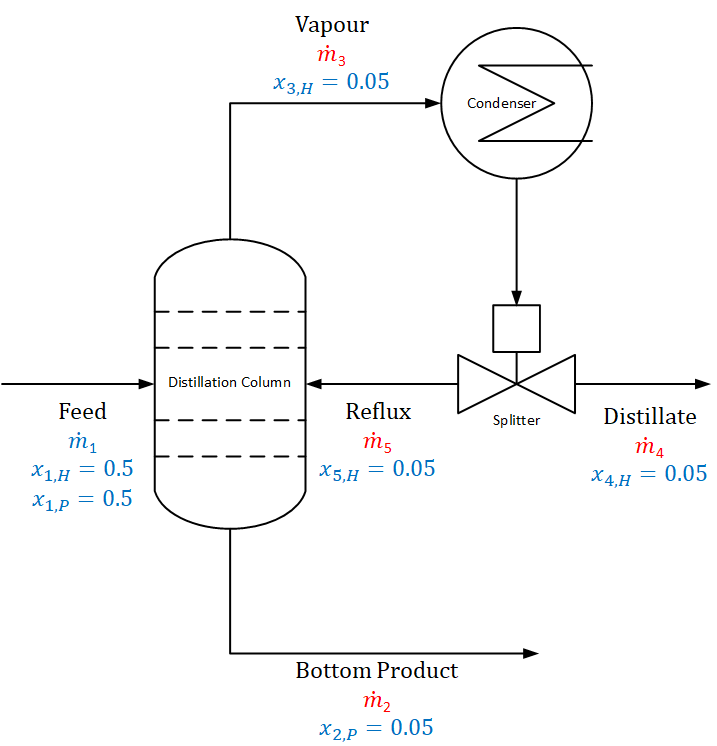
As shown in the figure above, hexane (C6H14, H) and pentane (C5H12, P) are separated in a distillation column. In this distillation process, some of the vapour (stream 3) is returned to the distillation column after condensation to control the temperature and solute concentration. The ratio of this recycle sub-stream (stream 4) to the top product (stream 4) is called reflux ratio (reflux ratio = Reflux/Distillate = R/D). The mass flow rate of the feed stream is 1 = 413 kg/h, the feed contains 50 wt.% hexane (x1,H = 0.5), the distillate is 5 wt.% hexane (x4,H = 0.05), the bottom stream is 5 wt.% pentane (x2,P = 0.05), and reflux ratio is 0.60 (5 / 4 = 0.6).
The atomic masses are: H2 = 2 g/mol and C = 12 g/mol.
The densities are: hexane = 655 kg/m and pentane = 626 kg/m
Q1.Determine the mass flow rate of the bottom product, i.e. 2, in kg/h to 1 decimal place.
Q2.Determine the mass flow rate of the reflux stream, i.e. 5, in kg/h to 1 decimal place.
Q3.Determine the mass flow rate of the vapour stream, i.e. 3, in kg/h to 1 decimal place.
Q4.Determine the mole flow rate of the vapour stream, i.e. 3, in kmol/h to 1 decimal place.
Vapour m3 X3,1 = 0.05 Condenser Distillation Column Feed m1 Splitter Reflux M , Distillate m4 X5 H = 0.05 X1,1 = 0.5 *1.p = 0.5 H X1,P X4,H = 0.05 - Bottom Product M = X2, P = 0.05Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


