Question
At time 1-0, the wavefunction for Hydrogen atom is v (r.0)=(200* (20/1.00 +2.00 + 2/211+31-1) where the subscripts are values of the quantum numbers
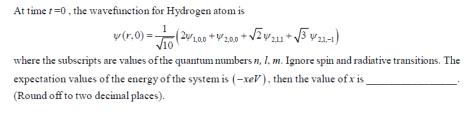
At time 1-0, the wavefunction for Hydrogen atom is v (r.0)=(200* (20/1.00 +2.00 + 2/211+31-1) where the subscripts are values of the quantum numbers n, 1. m. Ignore spin and radiative transitions. The expectation values of the energy of the system is (-xeV), then the value of x is (Round off to two decimal places).
Step by Step Solution
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantum Chemistry
Authors: Ira N. Levine
7th edition
321803450, 978-0321803450
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



