Question: b. The isomeric structures 3 and 4, MF C,H,O2, give rise to the 'H NMR spectra shown in Fig. 1 and Fig. 2. 9 m
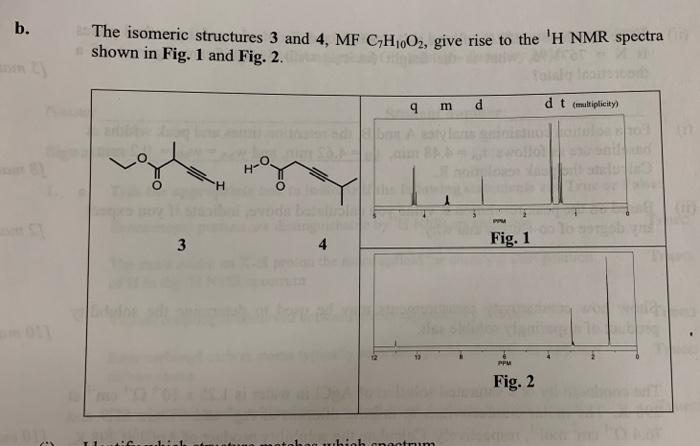

b. The isomeric structures 3 and 4, MF C,H,O2, give rise to the 'H NMR spectra shown in Fig. 1 and Fig. 2. 9 m d d t (multiplicity O " 3 4 Fig. 1 PPU Fig. 2 (i) Identify which structure matches which spectrum. (ii) Use a splitting tree to explain the origin of the quartet (q) signal at 4.20 ppm in Fig. 1. Include in your answer an explanation for the fact that the 4 lines of the quartet have relative intensities 1:3:3:1. (iii) Predict the relative integrals of the signals on the spectrum in Fig. 1. (iv) Explain the downfield origin of the signal at -11 ppm in Fig. 2 and account for the fact that this signal moves upfield when a spectrum of the same compound is recorded at lower concentration. (v) The signals in Fig. 2 are less well resolved, assuming it is a first order spectrum, predict the multiplicity of these signals. (vi) Draw and label a diagram of how you would expect the 'H'H COSY spectrum of compound 3 to appear
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


