Answered step by step
Verified Expert Solution
Question
1 Approved Answer
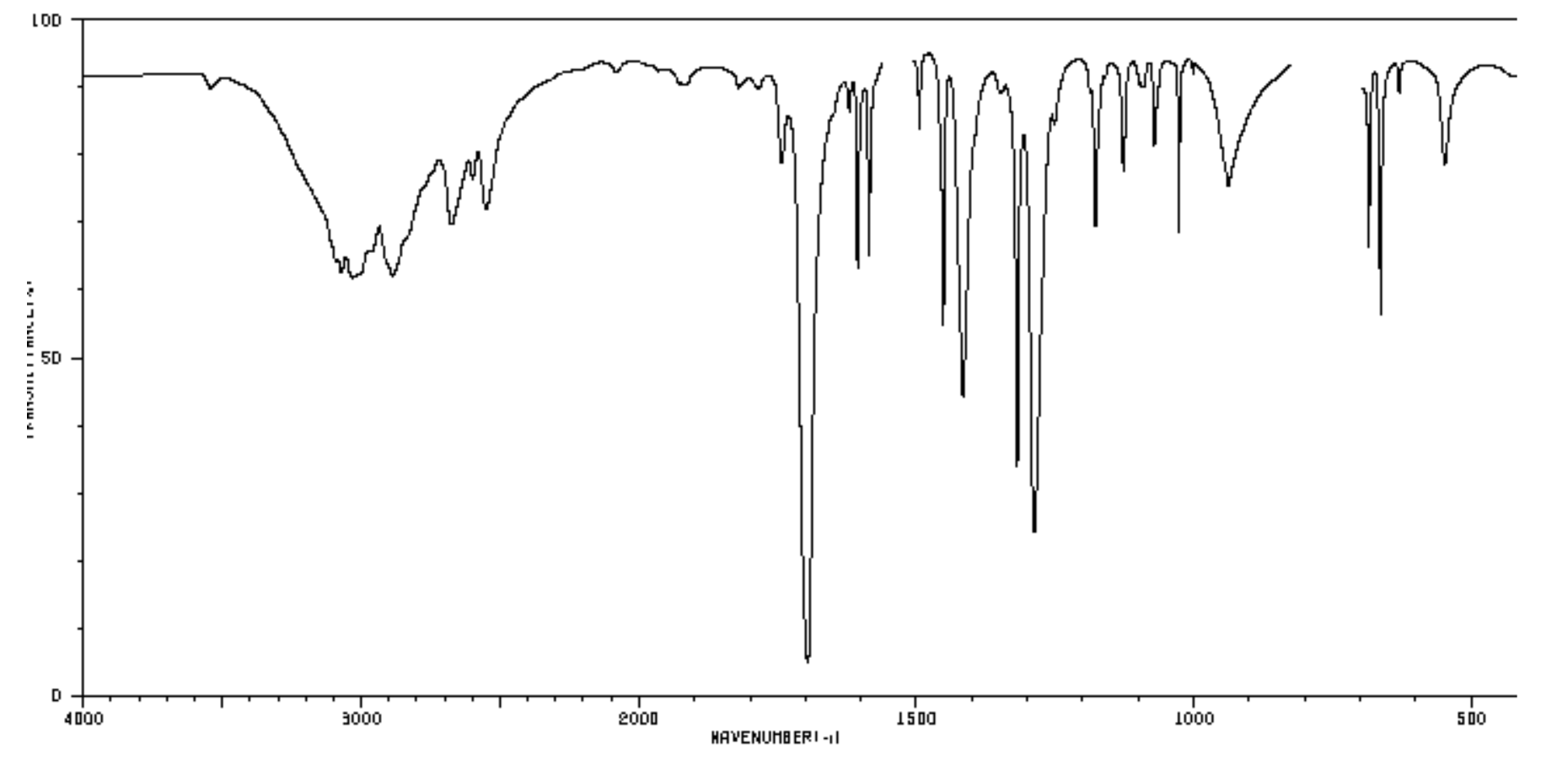
Based on analysis of the IR spectra for each unknown compound determine the absorption bands with wavenumber, intensity, and shape. Do for both regions. Intensity
Based on analysis of the IR spectra for each unknown compound determine the absorption bands with wavenumber, intensity, and shape. Do for both regions. Intensity can be described as strong, medium or weak. Shape can be described as sharp or broad.
For example: The IR spectrum of liquid unknown 1 exhibited a strong, broad absorption band at 3200 cm-1 and a weak but sharp absorption band at 2975 cm-1. Other notable absorption bands in the IR spectrum for 1 were at 1000 and 950 cm-1.
Carboxylic acid unknown solid
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


