Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate solubility of calcium hydroxide in each of the solutions. Only need to determine Ksp of calcium hydroxide in water solution. Average solubilities in g/L
Calculate solubility of calcium hydroxide in each of the solutions. Only need to determine Ksp of calcium hydroxide in water solution. 
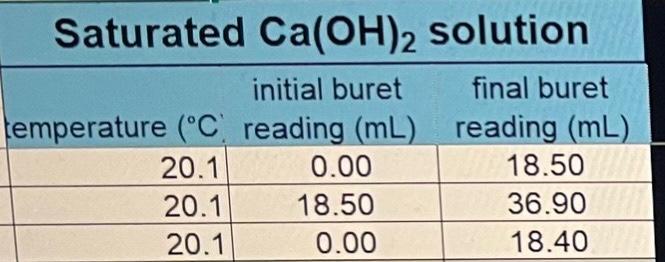
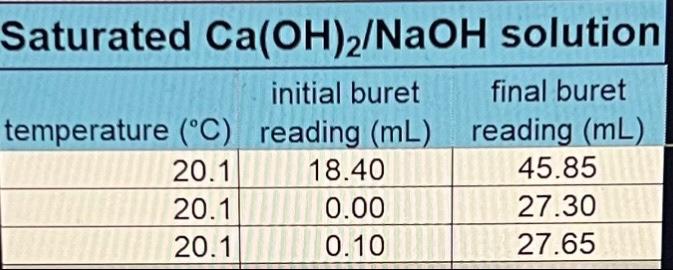
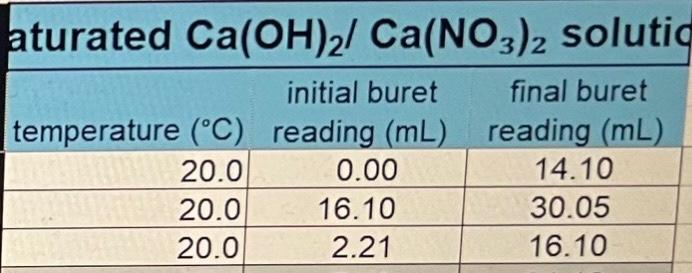
ConcentrationofstandardizedHClVolumeofCa(OH)2solutionofNaOHinCa(OH)2/NaOHsolutionO3)2inCa(OH)2/Ca(NO3)2solution0.0648M25.00mL0.0510M0.0208M Saturated Ca(OH)2 solution initial buret final buret lemperature (C. reading (mL) reading (mL) \begin{tabular}{|r|r|r|} \hline 20.1 & 0.00 & 18.50 \\ \hline 20.1 & 18.50 & 36.90 \\ \hline 20.1 & 0.00 & 18.40 \\ \hline \end{tabular} Saturated Ca(OH)2/NaOH solution initial buret final buret temperature (C) reading (mL) reading (mL) \begin{tabular}{|l|r|l|} \hline 20.1 & 18.40 & 45.85 \\ \hline 20.1 & 0.00 & 27.30 \\ \hline 20.1 & 0.10 & 27.65 \\ \hline \end{tabular} Average solubilities in g/L and mol/L (table form) to include class and individual data with standard deviations.
Average Ksp value in water (table form again)
Explain how you arrived at these Ksp and solubilty results please for understanding.
Data:




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


