Answered step by step
Verified Expert Solution
Question
1 Approved Answer
- Chemical Reaction Engineering problem - It is a Semi-batch reactor problem. Please upload the graph using Excel Please write neatly so that I can
- Chemical Reaction Engineering problem -
It is a Semi-batch reactor problem.
 Please upload the graph using Excel
Please upload the graph using Excel
Please write neatly so that I can read it easily
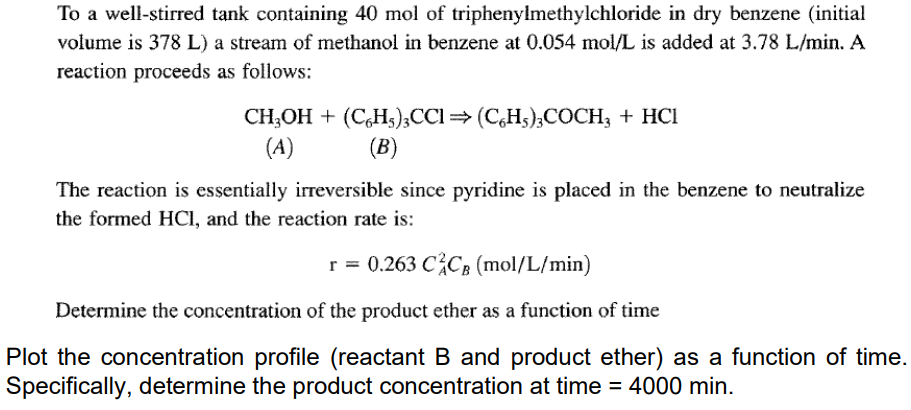
To a well-stirred tank containing 40mol of triphenylmethylchloride in dry benzene (initial volume is 378L ) a stream of methanol in benzene at 0.054mol/L is added at 3.78L/min. A reaction proceeds as follows: CH3OH+(C6H5)3CCl(C6H5)3COCH3+HCl (A) (B) The reaction is essentially irreversible since pyridine is placed in the benzene to neutralize the formed HCl, and the reaction rate is: r=0.263CA2CB(mol/L/min) Determine the concentration of the product ether as a function of time Iot the concentration profile (reactant B and product ether) as a function of time pecifically, determine the product concentration at time =4000minStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


