Answered step by step
Verified Expert Solution
Question
1 Approved Answer
complete question The figure shows a series of standard additions of Cu?+ to acidified tap water measured by anodic stripping voltammetry at an iridium electrode.
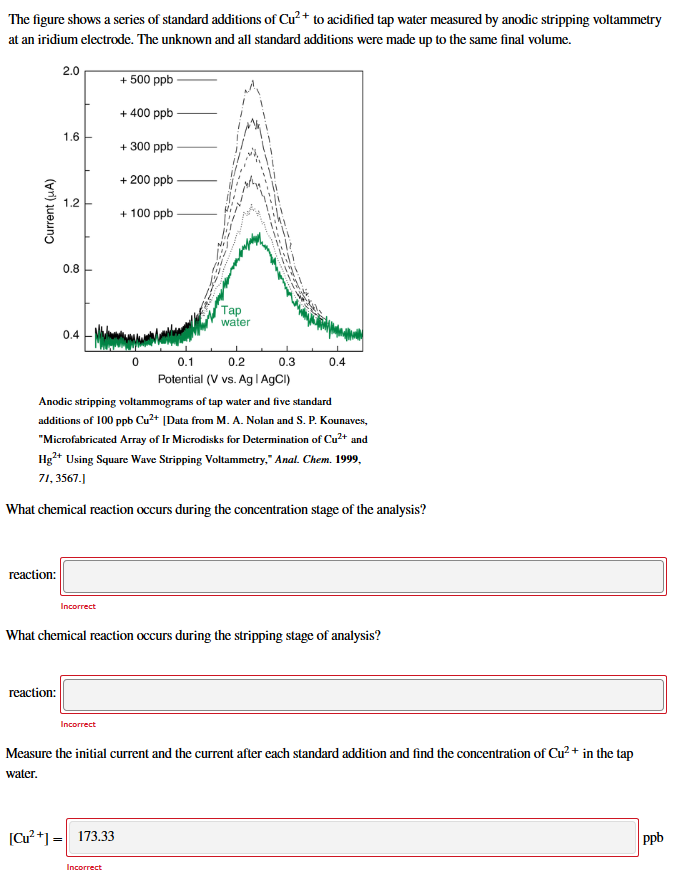
complete question
The figure shows a series of standard additions of Cu?+ to acidified tap water measured by anodic stripping voltammetry at an iridium electrode. The unknown and all standard additions were made up to the same final volume. 2.0 + 500 ppb +400 ppb 1.6 + 300 ppb +200 ppb 1.2 Current (A) + 100 ppb 0.8 Tap water 0.4 0.1 0.2 0.3 0.4 Potential (V vs. Ag | AgCl) Anodic stripping voltammograms of tap water and five standard additions of 100 ppb Cu2+ [Data from M. A. Nolan and S.P. Kounaves, "Microfabricated Array of Ir Microdisks for Determination of Cu2+ and Hg2+ Using Square Wave Stripping Voltammetry," Anal. Chem. 1999, 71, 3567.) What chemical reaction occurs during the concentration stage of the analysis? reaction: Incorrect What chemical reaction occurs during the stripping stage of analysis? reaction: Incorrect Measure the initial current and the current after each standard addition and find the concentration of Cu2+ in the tap water. [Cu+1 = 173.33 ppb IncorrectStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


