Question
Consider the following spectrum of a diatomic molecule, obtained at 305.00K. Use the information contained in this spectrum to answer the different parts of this
Consider the following spectrum of a diatomic molecule, obtained at 305.00K.
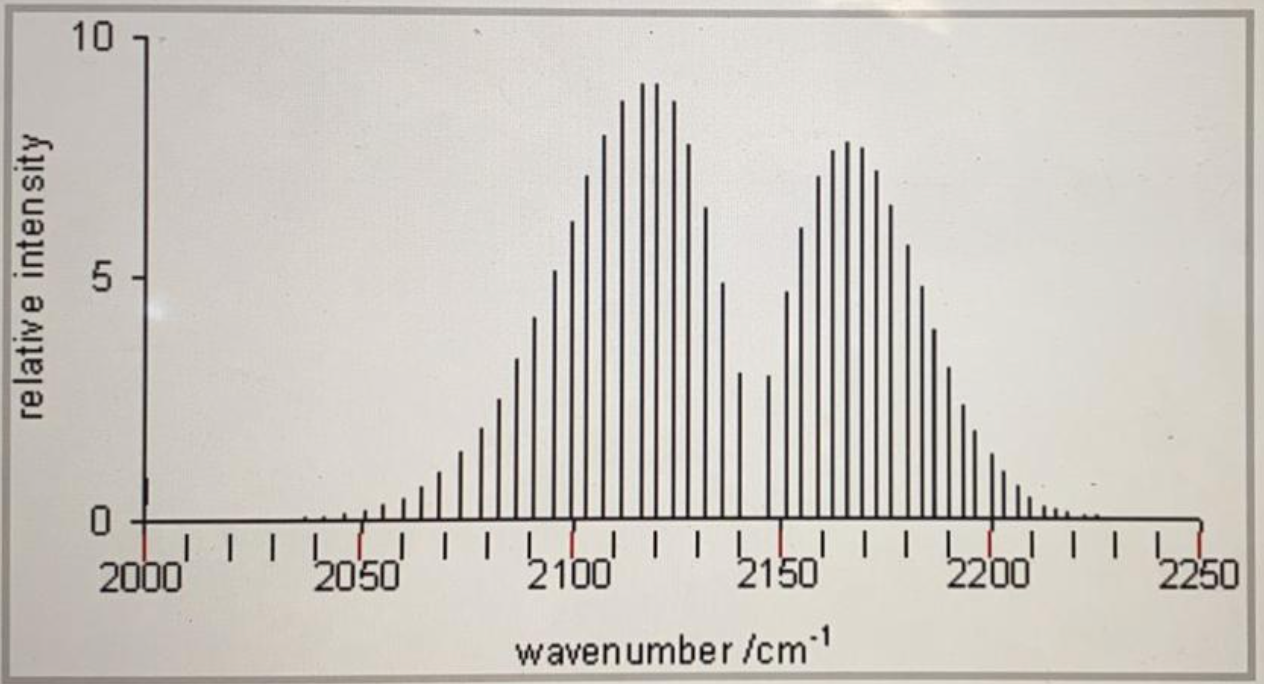 Use the information contained in this spectrum to answer the different parts of this problem. The reduced mass of this molecule is =1.1031 x 10-26 kg. Calculate r, the bond length of this molecule. Calculate the ratio n J=3/ n J=0 for a temperature of T=305.00 K. Why is there no line (peak) at position 2145 cm-1 in this spectrum?
Use the information contained in this spectrum to answer the different parts of this problem. The reduced mass of this molecule is =1.1031 x 10-26 kg. Calculate r, the bond length of this molecule. Calculate the ratio n J=3/ n J=0 for a temperature of T=305.00 K. Why is there no line (peak) at position 2145 cm-1 in this spectrum?
1. The bond length of this molecule is r= ___________m
2. For this molecule, at the given temperature n J=3/ n J=0 = _______________
The reason there is no line (peak) at position 2145 cm-1 in this spectrum is ______________________
10 relative intensity 5 0 2000 IT 2050 2100 2150 2200 2250 wavenumber /cmStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


