Answered step by step
Verified Expert Solution
Question
1 Approved Answer
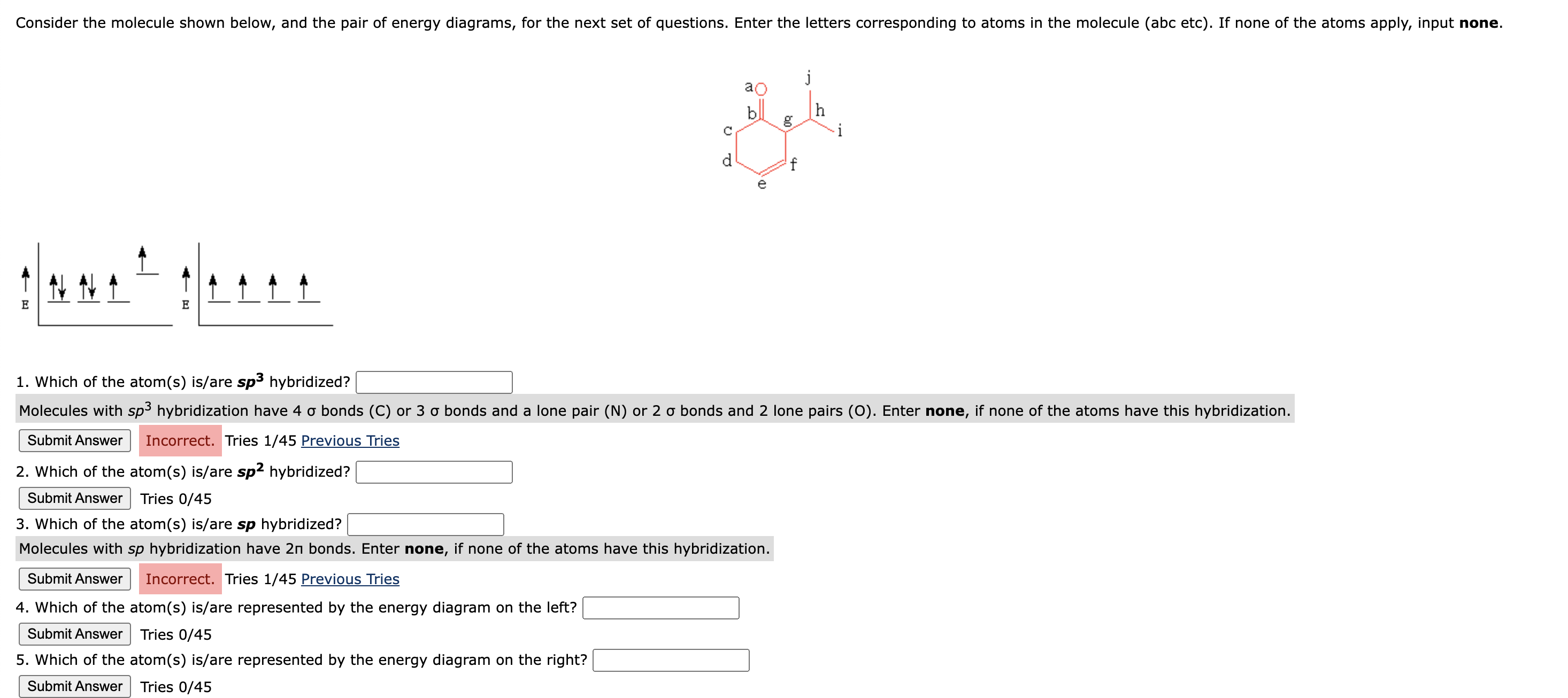
Consider the molecule shown below, and the pair of energy diagrams, for the next set of questions. Enter the letters corresponding to atoms in the
Consider the molecule shown below, and the pair of energy diagrams, for the next set of questions. Enter the letters corresponding to atoms in the molecule abc etc If none of the atoms apply, input none.
Which of the atoms isare sp hybridized?
Molecules with sp hybridization have sigma bonds C or sigma bonds and a lone pair N or sigma bonds and lone pairs O Enter none, if none of the atoms have this hybridization.
Incorrect. Tries Previous Tries
Which of the atoms isare sp hybridized?
Tries
Which of the atoms isare sp hybridized?
Molecules with sp hybridization have pi bonds. Enter none, if none of the atoms have this hybridization.
Incorrect. Tries Previous Tries
Which of the atoms isare represented by the energy diagram on the left?
Tries
Which of the atoms isare represented by the energy diagram on the right?
Tries
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


