Question: Data showing the standard molar constant-pressure heat capacity Com for water vapor as a function of temperature is provided in the Excel file Heat Capacity
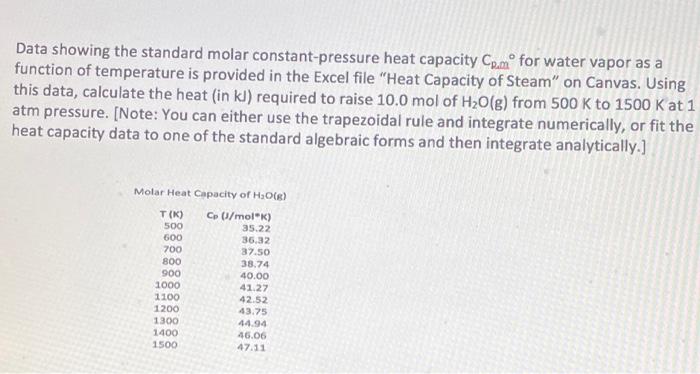
Data showing the standard molar constant-pressure heat capacity Com for water vapor as a function of temperature is provided in the Excel file "Heat Capacity of Steam" on Canvas. Using this data, calculate the heat (in kl) required to raise 10.0 mol of H2O(g) from 500 K to 1500 K at 1 atm pressure. [Note: You can either use the trapezoidal rule and integrate numerically, or fit the heat capacity data to one of the standard algebraic forms and then integrate analytically.] Molar Heat Capacity of H (6) T(K) 500 600 700 800 900 1000 1100 1200 1300 1400 1500 Cp (l/mol) 35.22 36.32 37.50 38.74 40.00 41.27 42.52 43.75 44.94 46.06 47.11
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


