Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine the heating value (LHV and HHV) of C12H26 (liquid) when burned with 25% excess air. Air and fuel enter at 25C, and products leaves
Determine the heating value (LHV and HHV) of C12H26 (liquid) when burned with
25% excess air. Air and fuel enter at 25ºC, and products leaves at 900 K. Assume
P =101 KPa. Please refer to the images if it helps.
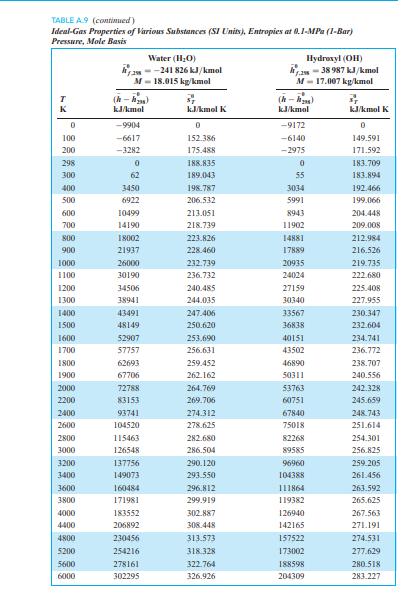
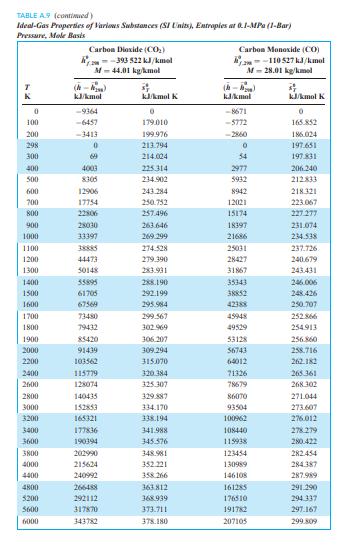
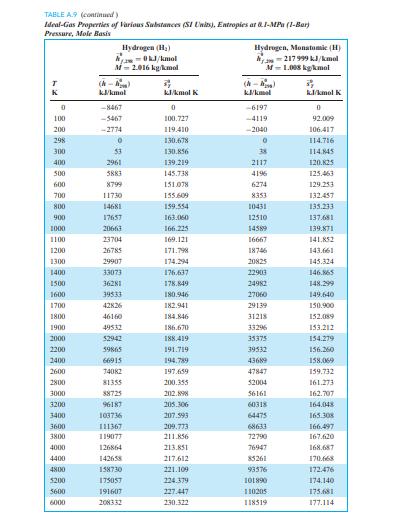
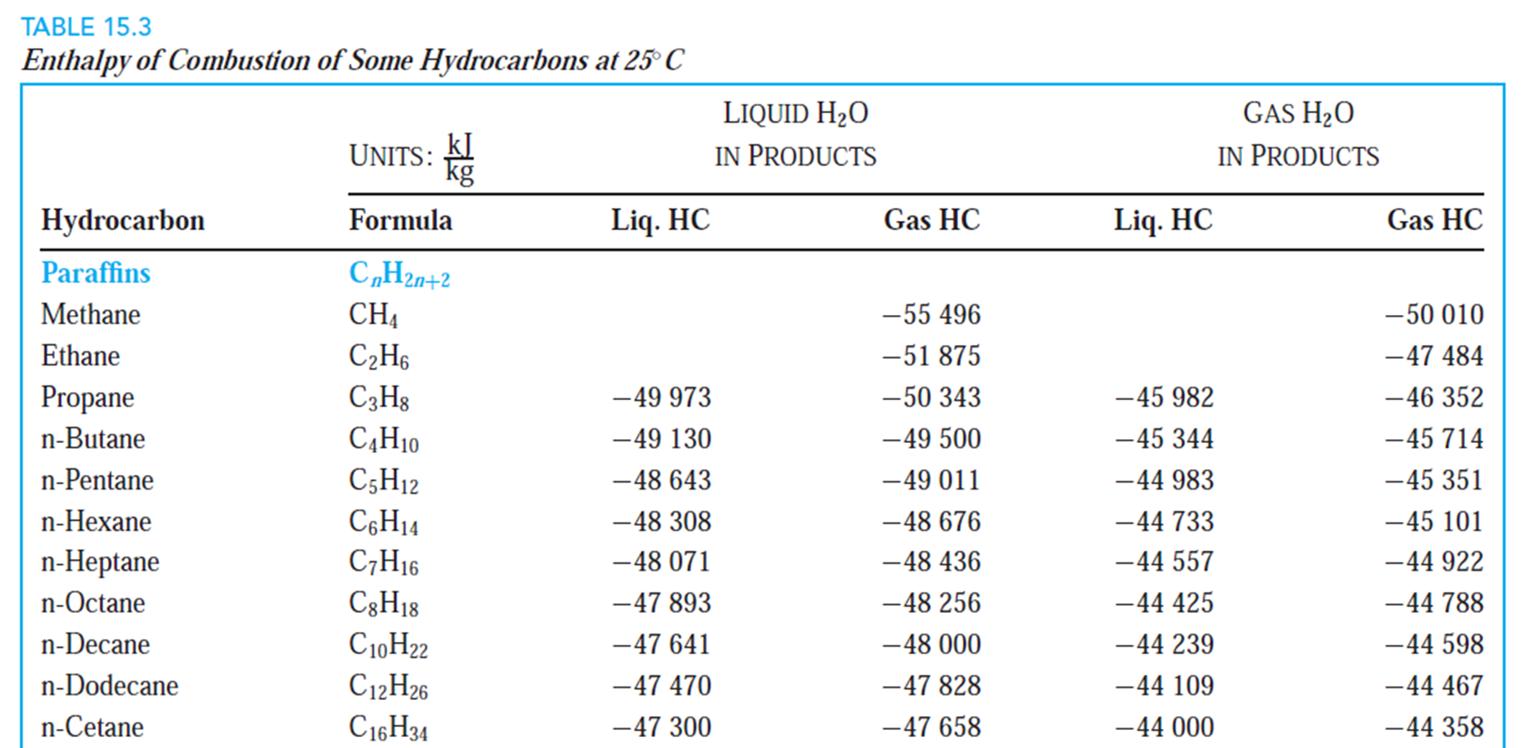
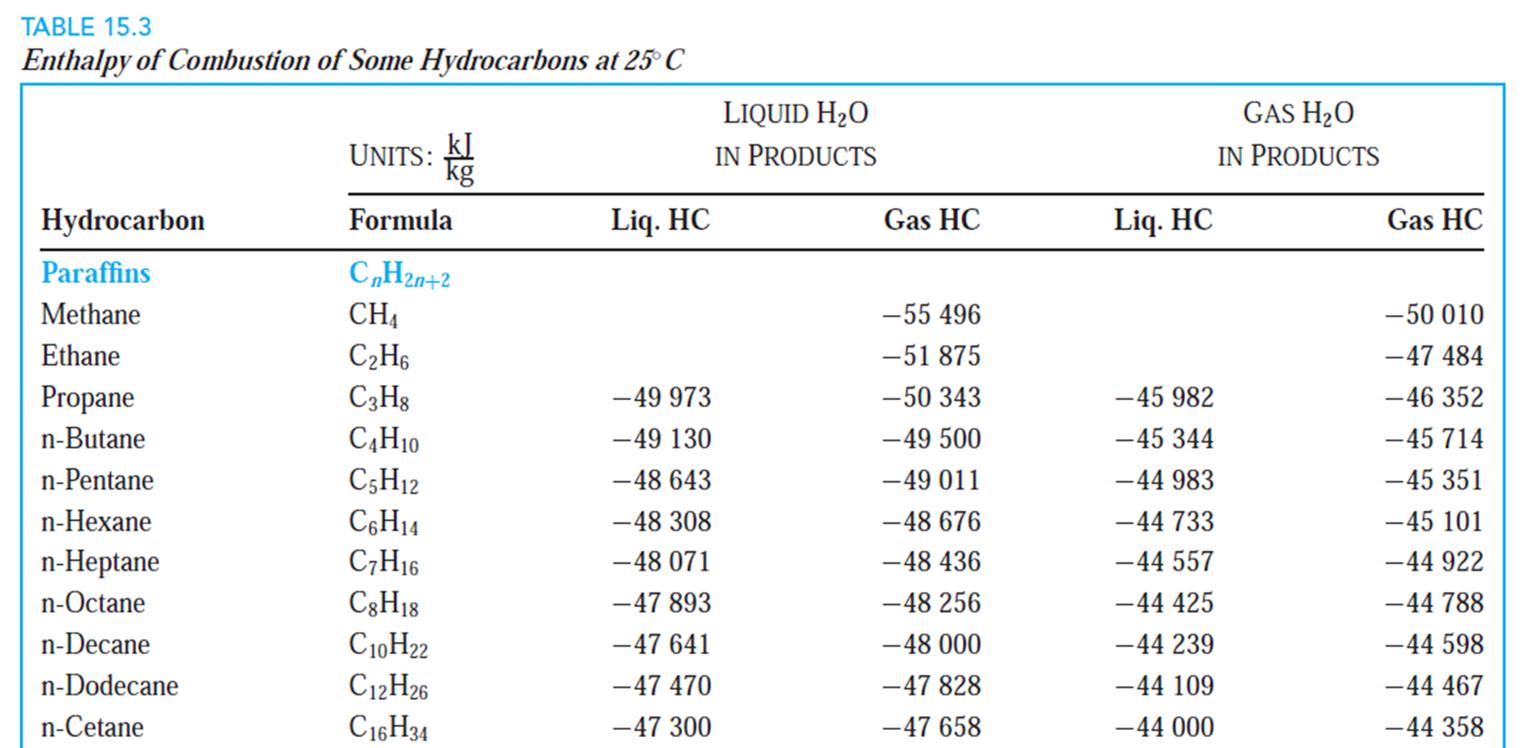
TABLE A9 (continued) Ideal-Gas Properties of Various Substances (SI Units), Entropies at 0.1-MPa (1-Bar) Pressure, Mole Basis Water (H2O) --241 826 kJ/kmol Hydroxyl (OH) am-38 987 kJ/kmol M-18.015 kg/kmol M-17.007 kg/kmol TR (-) (-) K kJ/kmol kJ/kmol K kJ/kmol kJ/kmol K 0 -9904 0 -9172 0 100 -6617 152.386 -6140 149.591 200 -3282 175.4881 -2975 171.592 298 0 188.8351 0 183.709 300 62 189.043 55 183.894 400 3450 198.787 3034 192.466 500 6922 206.532 5991 199.066 600 10499 213.051 8943 204.448 700 14190 218.739 11902 209.008 800 18002 223.826 14881 212.984 900 21937 228.460 17889 216.526 1000 26000 232.739 20935 219.735 1100 30190 236.732 24024 222.680 1200 34506 240.485 27159 225.408 1300 38941 244.035 30340 227.955 1400 43491 247.406 33567 230.347 1500 48149 250.620 36838 232.604 1600 52907 253.690 40151 234.741 1700 57757 256.631 43502 236.772 1800 62693 259.452 46890 238.707 1900 67706 262.162 50311 240.556 2000 72788 264.769 53763 242.328 2200 83153 269.706 60751 245.659 2400 93741 274.312 67840 248.743 2600 104520 278.625 75018 251.614 2800 115463 282.680 82268 254.301 3000 126548 286.504 89585 256.825 3200 137756 290.120 96960 259.205 3400 149073 293.550 104388 261.456 3600 160484 296.812 111864 263.592 3800 171981 299.919 119382 265.625 4000 183552 302.887 126940 267.563 4400 206892 308.448 142165 271.191 4800 230456 313.573 157522 274.531 5200 254216 318.328 173002 277.629 5600 278161 322.764 188598 280.518 6000 302295 326.926 204309 283.227
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


