Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine van't Hoff factor for calcium chloride. Record in LabData. Volume of DI water (mL) Mass of calcium chloride (g) Moles of calcium chloride (mol)
Determine van't Hoff factor for calcium chloride. Record in LabData.
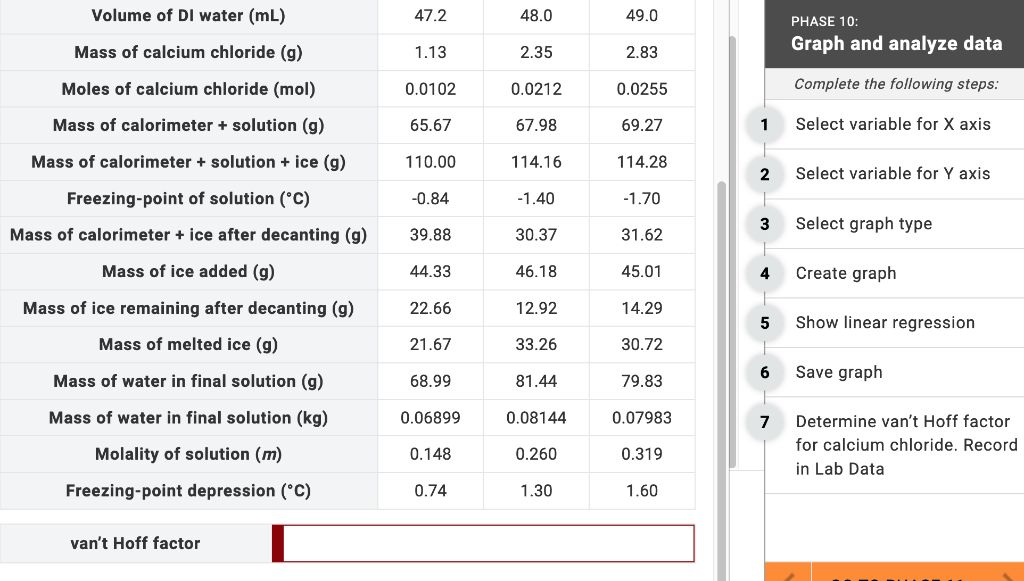
Volume of DI water (mL) Mass of calcium chloride (g) Moles of calcium chloride (mol) Mass of calorimeter + solution (g) Mass of calorimeter + solution + ice (g) Freezing-point of solution (C) Mass of calorimeter + ice after decanting (g) Mass of ice added (g) Mass of ice remaining after decanting (g) Mass of melted ice (g) Mass of water in final solution (g) Mass of water in final solution (kg) Molality of solution (m) Freezing-point depression (C) van't Hoff factor 47.2 1.13 0.0102 65.67 110.00 -0.84 39.88 44.33 22.66 21.67 68.99 0.06899 0.148 0.74 48.0 2.35 0.0212 67.98 114.16 -1.40 30.37 46.18 12.92 33.26 81.44 0.08144 0.260 1.30 49.0 2.83 0.0255 69.27 114.28 -1.70 31.62 45.01 14.29 30.72 79.83 0.07983 0.319 1.60 1 2 3 5 7 PHASE 10: Graph and analyze data Complete the following steps: Select variable for X axis Select variable for Y axis Select graph type Create graph Show linear regression. Save graph Determine van't Hoff factor for calcium chloride. Record in Lab Data
Step by Step Solution
★★★★★
3.35 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
The Vant Hoff factor is ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


