
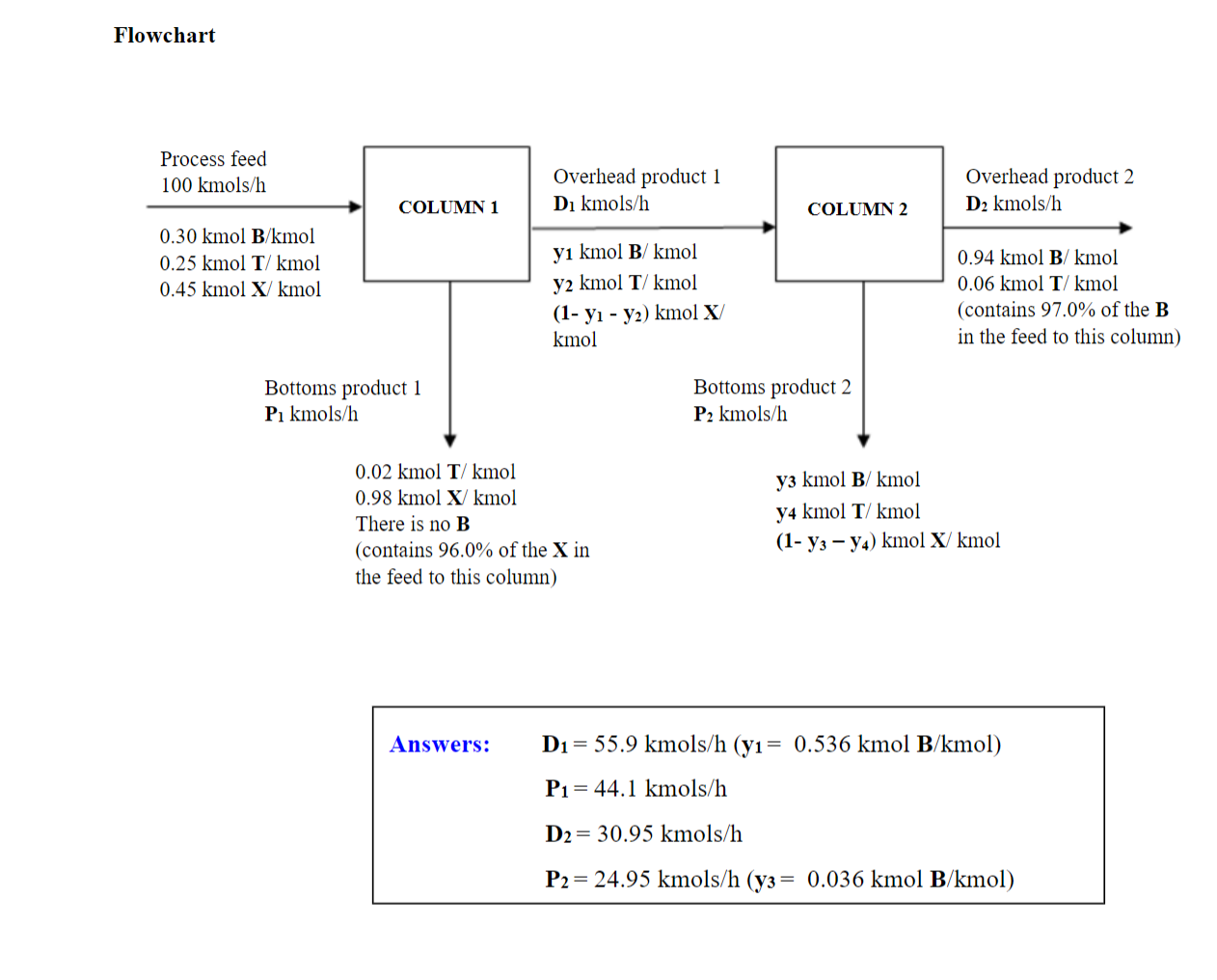
Due Feb. 13 (Monday) 11:50 pm, on Canvas. Please submit the solution for the Homework as a single PDF file. Files submitted in any other format will not be graded. Make sure that your PDF file contains solution for all the problems before uploading because once the due date has passed, missing pages or incorrect page scans will not be considered for grading. ALL SOLUTIONS MUST BE HANDWRITTEN. Only 1 problem for 100 points. Solve the problems as instructed. 1. The logic in each step of your solutions should be clearly seen. 2. If you use information not from the problem statement, give your source. 3. If you have other assumptions aside from those given, write them down. Distillation with multiple units One hundred kilomoles per hour of a liquid mixture (the process feed) containing 30.0mole% benzene (B), 25.0\% toluene (T), and the balance xylene (X) is fed to a distillation column. The bottoms product contains 98.0 mole %X and no B, and 96.0% of the X in the feed is recovered in this stream. The overhead product is fed to a second column. The overhead product from the second column contains 97.0% of the B in the feed to this column. The composition of this stream is 94.0 mole %B and the balance T. Assume that the process is continuous and at steady-state. a) Is the flowchart given below a completely labeled one? Why or why not? b) Identify all the system(s) where Material Balances can be made and then write all the possible Material Balance equations for each. (Hint: There should be three systems.) c) For each system, do a degree-of-freedom analysis. (Hint: The equations will be from material balances and other equations from the given. For example, for COLUMN 1, you are given that 96.0% of the X in the Process feed is recovered in the bottoms product (P1). In equation form, this is: Moles X in bottoms product =0.96 Moles X in Process feed (0.98kmolX/kmol)(P1kmols/h)=(0.96)(0.45kmolX/kmol)(100kmols/h)) d) Which system will you use first to start solving for the unknowns? Why? Describe how you will go about solving the unknowns for this system. e) Solve for all the unknowns (flowrates and mole fractions). Flowchart Answers: D1=55.9kmols/h(y1=0.536kmolB/kmol)P1=44.1kmols/hD2=30.95kmols/hP2=24.95kmols/h(y3=0.036kmolB/kmol)








