Question
An aqueous solution of cobalt(II) iodide, CoI2, is made by dissolving 25.1 grams of cobalt(II) iodide in sufficient water in a 500. mL volumetric
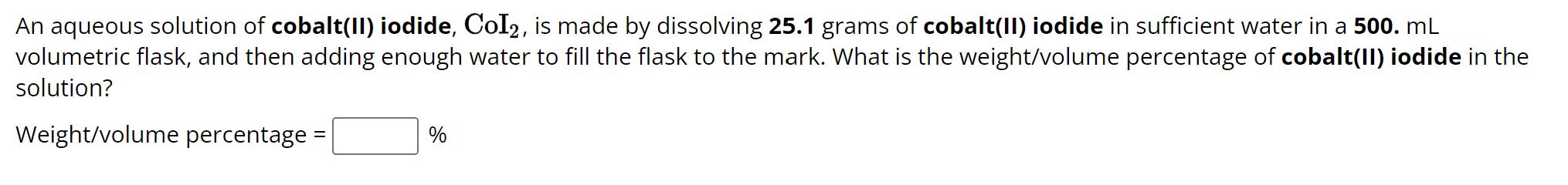
An aqueous solution of cobalt(II) iodide, CoI2, is made by dissolving 25.1 grams of cobalt(II) iodide in sufficient water in a 500. mL volumetric flask, and then adding enough water to fill the flask to the mark. What is the weight/volume percentage of cobalt(II) iodide in the solution? Weight/volume percentage= %
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer So the weightvolume percentage of cobaltII iodide in the solu...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Accounting
Authors: David Spiceland, Wayne M. Thomas, Don Herrmann
5th edition
1259914895, 978-1259914898
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



