Answered step by step
Verified Expert Solution
Question
1 Approved Answer
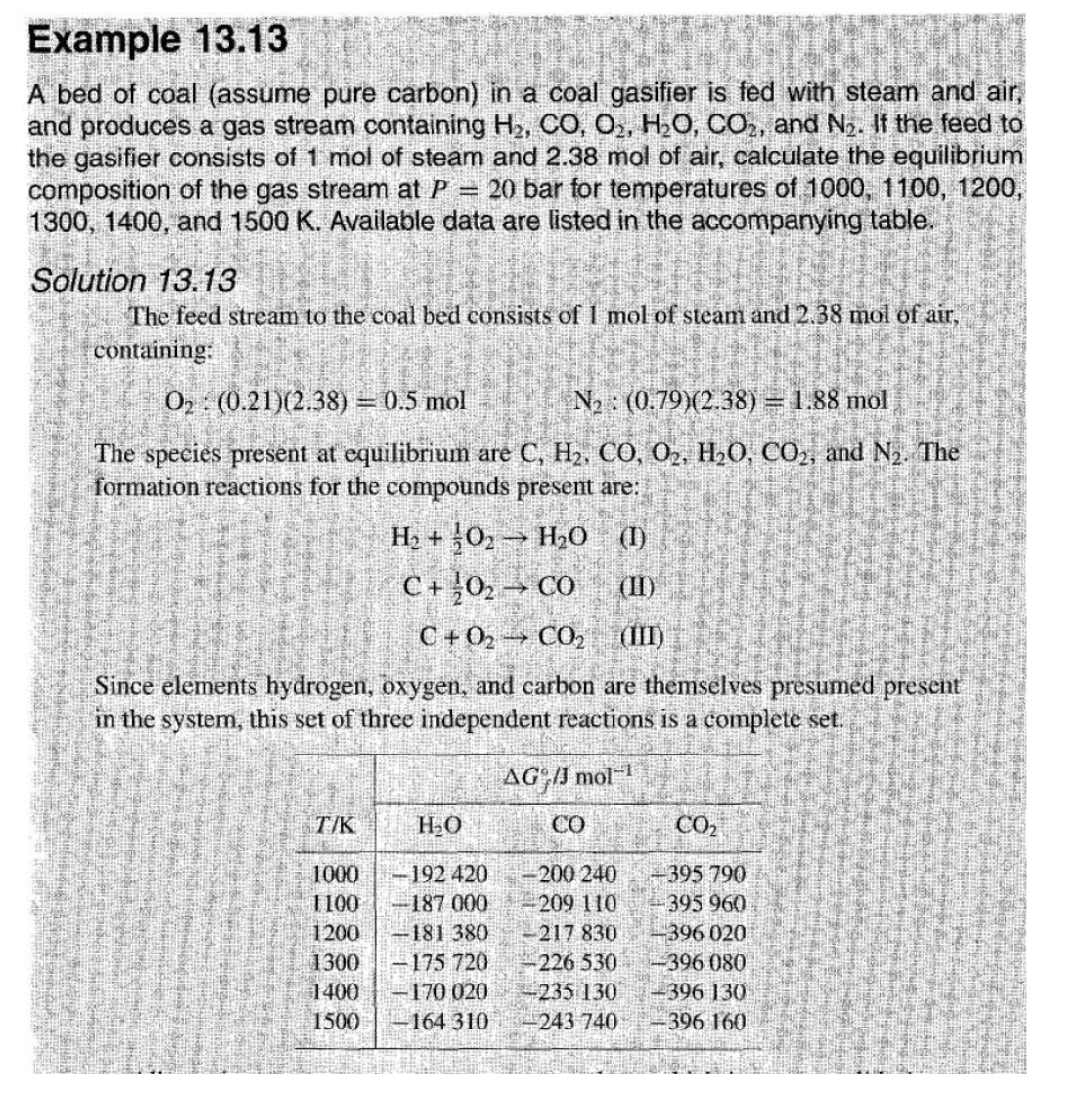
Example 1 3 . 1 3 A bed of coal ( assume pure carbon ) in a coal gasifier is fed with steam and air,
Example
A bed of coal assume pure carbon in a coal gasifier is fed with steam and air,
and produces a gas stream containing and If the feed to
the gasifier consists of mol of steam and mol of air, calculate the equilibrium
composition of the gas stream at bar for temperatures of
and Available data are listed in the accompanying table.
Solution
The feed stream to the coal bed consists of mol of steam and mol of air,
containing:
:mol,:mol
The species present at equilibrium are and The
formation reactions for the compounds present are:
Since elements hydrogen, oxygen, and carbon are themselves presumed present
in the system, this set of three independent reactions is a complete set.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


