Question
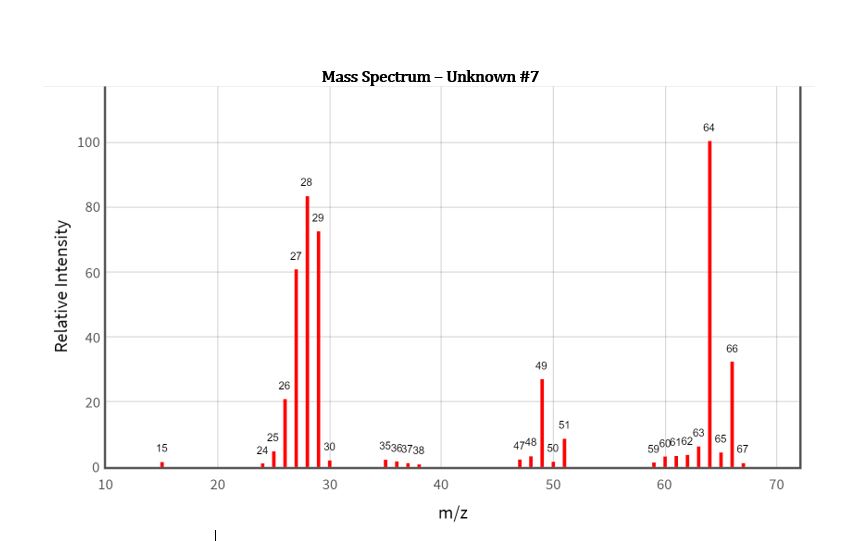
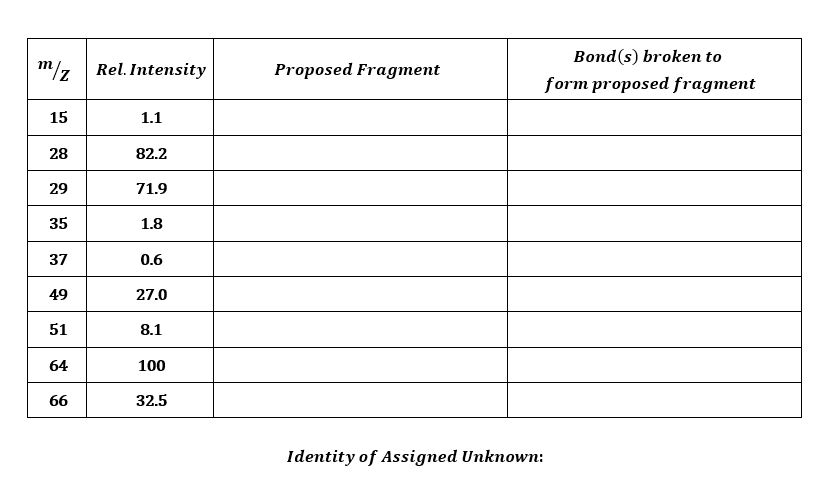
Experiment 3 Mass Spectrometry In this dry-lab, you must identify the unknown organic compound in your assigned mass spectrum, as well as identifying the listed
Experiment 3 Mass Spectrometry
In this dry-lab, you must identify the unknown organic compound in your assigned mass spectrum, as well as identifying the listed fragments in the table.
All unknowns contain C and H, and may contain either N, O, Cl, or Br.
Unknowns cannot contain multiple different non-C/H atoms, but might contain two of the same non-C/H atoms.
i.e. an unknown cannot contain both O and Br, but could contain two Br atoms.
Fragments in your mass spectrum will be the result of breaking bonds in the parent unknown molecule. Formation of new bonds that were not present in the original compound is extremely rare.
The following isotopic ratios might be useful for analysing your spectrum:
Hydrogen: 1H (100) : 2H (0.016) 2H can be completely neglected
Carbon: 12C (100) : 13C (1.11)
Nitrogen: 14N (100) : 15N (0.38) 15N can be completely neglected
Oxygen: 16O (100) : 17O (0.04) 17O can be completely neglected
Chlorine: 35Cl (100) : 37Cl (32.5)
Bromine: 79Br (100) : 81Br (98)
Questions to ask as you work through your spectrum:
Do you have peaks at m/Z = 35 and 37 in a ~3:1 ratio?
If so, do you have three heavy peaks in a ~9:6:1 ratio?
Do you have peaks at m/Z = 79 and 81 in a ~1:1 ratio?
If so, do you have three heavy peaks in a ~1:2:1 ratio?
Do you have peaks at m/Z = 14 or 15? What organic fragments could these be? (hint: they do not contain nitrogen)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


