Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Figure 1 shows a two-stage distillation process for the separation of a propylene-propane mixture to produce 99.5 mol% propylene and 96 mol% propane. 1000 kmol/h
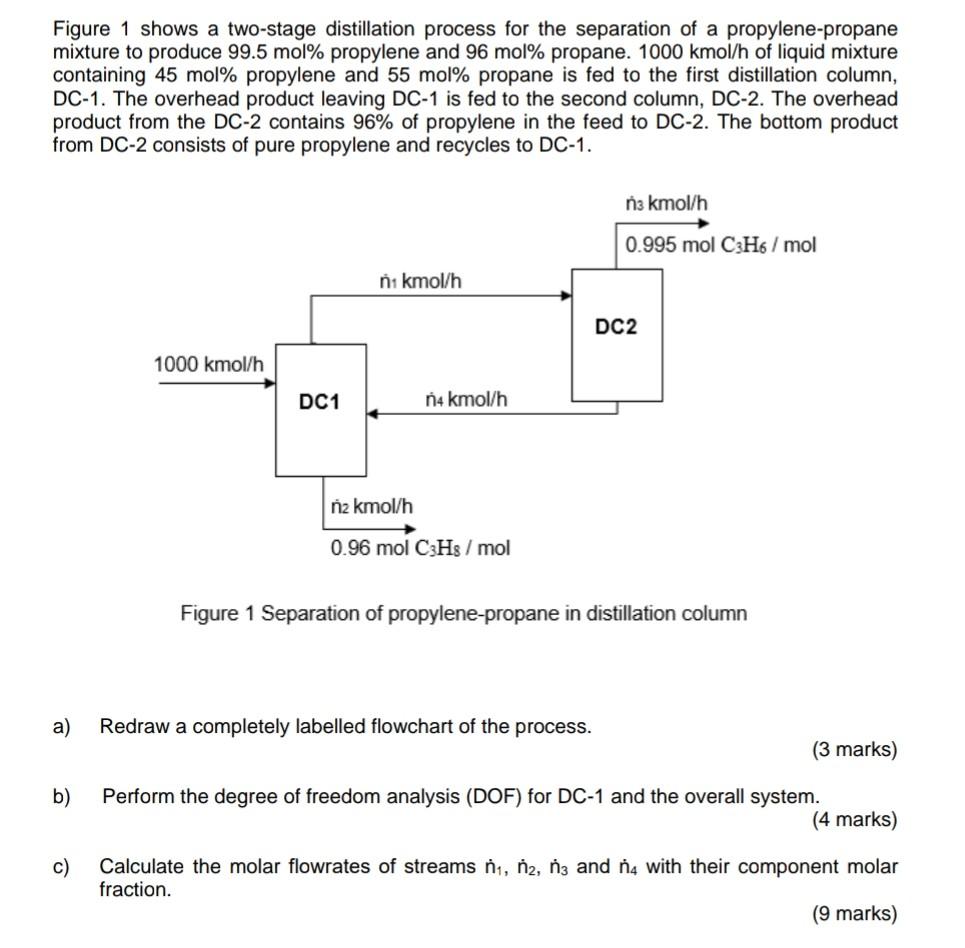
Figure 1 shows a two-stage distillation process for the separation of a propylene-propane mixture to produce 99.5 mol% propylene and 96 mol% propane. 1000 kmol/h of liquid mixture containing 45 mol% propylene and 55 mol% propane is fed to the first distillation column, DC-1. The overhead product leaving DC-1 is fed to the second column, DC-2. The overhead product from the DC-2 contains 96% of propylene in the feed to DC-2. The bottom product from DC-2 consists of pure propylene and recycles to DC-1. n3 kmol/h 0.995 mol C3H6/mol na kmol/h DC2 1000 kmol/h DC1 n4 kmol/h n2 kmol/h 0.96 mol CH3 /mol Figure 1 Separation of propylene-propane in distillation column a) Redraw a completely labelled flowchart of the process. (3 marks) b) Perform the degree of freedom analysis (DOF) for DC-1 and the overall system. (4 marks) c) Calculate the molar flowrates of streams na, na, n3 and nu with their component molar fraction. (9 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


