Answered step by step
Verified Expert Solution
Question
1 Approved Answer
fill in blanks 6. a. Complete the following table of Trial 1 (See Report Sheet.) for determining the molar volume of CO, and the per
fill in blanks 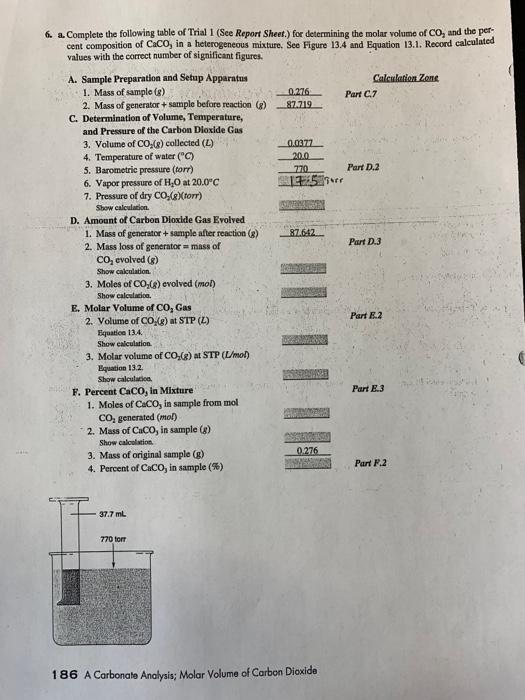
6. a. Complete the following table of Trial 1 (See Report Sheet.) for determining the molar volume of CO, and the per cent composition of Caco, in a heterogeneous mixture. See Figure 13.4 and Equation 13.1. Record calculated values with the correct number of significant figures. Calculation Zone Part 7 0.276 87.719 0.0377 200 770 17:59 Part D.2 87642 Part 1.3 A. Sample Preparation and Setup Apparatus 1. Mass of sample (8) 2. Mass of generator + sample before reaction () C. Determination of Volume, Temperature, and Pressure of the Carbon Dioxide Gas 3. Volume of CO (8) collected (L) 4. Temperature of water ("C) 5. Barometric pressure (torr) 6. Vapor pressure of H.O at 20.0C 7. Pressure of dry CO.()(tor) Showcaleaton D. Amount of Carbon Dioxide Gas Evolved 1. Mass of generator + sample after reaction() 2. Mass loss of generator = mass of CO, evolved (R) Show calculation 3. Moles of CO (8) evolved (mol) Show calculacice E. Molar Volume of CO, Gas 2. Volume of CO (8) at STP (L) Equatic 13.4. Show calculation 3. Molar volume of CO (2) At STP (L/mol) Equation 13.2 Show calculation F. Percent Caco, in Mixture 1. Moles of Caco, in sample from mol CO, generated (mol) 2. Mass of Coco, in sample (8) Show calculation 3. Mass of original sample (8) 4. Percent of Caco, in sample (%) Part 2 3 Part E.3 0276 2 Part 2 37.7 ml 770 torr 186 A Carbonate Analysis: Molar Volume of Carbon Dioxide 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


