Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Hello! This is CH 338 Diels-Alder Lab... we are finding how much diene is in eucalyptus oil. Empty funnel mass: 8.1001 g Funnel with crystal
Hello! This is CH 338 Diels-Alder Lab... we are finding how much diene is in eucalyptus oil.
Empty funnel mass: 8.1001 g
Funnel with crystal material after Buchner filtration: 8.3939 g
I need help filling out the table please and thank you!
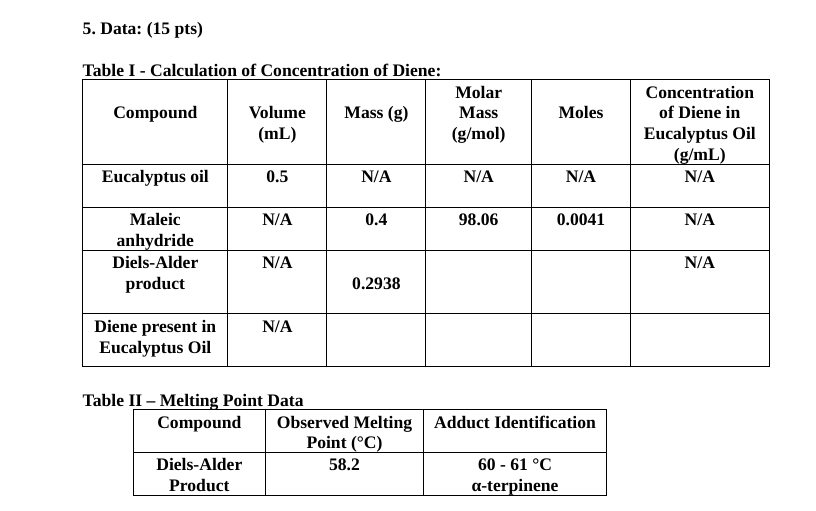
5. Data: (15 pts) Table I - Calculation of Concentration of Diene: Molar Compound Volume Mass (g) Mass (mL) (g/mol) Moles Concentration of Diene in Eucalyptus Oil (g/mL) N/A Eucalyptus oil 0.5 N/A N/A N/A N/A 0.4 98.06 0.0041 N/A Maleic anhydride Diels-Alder product N/A N/A 0.2938 N/A Diene present in Eucalyptus Oil Table II Melting Point Data Compound Observed Melting Adduct Identification Point (C) Diels-Alder 58.2 60 - 61 C Product a-terpineneStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


