Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help Data Table 1. Precipitation of Chloride inn Using your data and information in the General Chemistry 1 Loboratory Manual, calculate the following values. Show
help 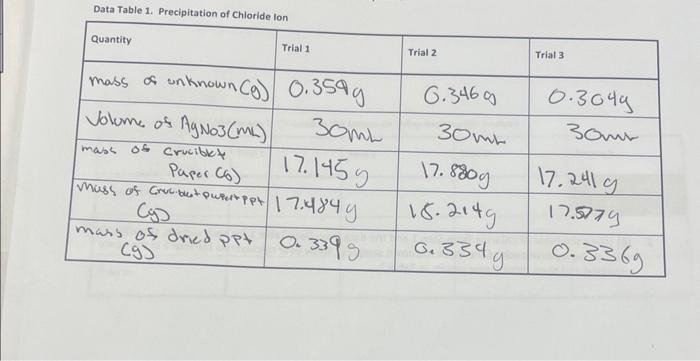

Data Table 1. Precipitation of Chloride inn Using your data and information in the General Chemistry 1 Loboratory Manual, calculate the following values. Show all steps of your work, be sure to use the proper number of significant figures and include all units in your calculations. 1. (22) In lab, you obtained the mass of the dried AgCl precipitate. Now, use stoichiometry calculations to obtain the mass of Cl in each of the three precipitates. Show your calculations for Trial 1 in the space provided and then record your results in Table 2. Note that the molar mass for silver is 107.87g/mol and the molar mass for chlorine is 35.45g/mol. 2. (6) Using the mass of chlorine from Step 1 and the data from Data Table 1 for each sample, calculate the % chloride by mass in each of the three unknown samples. Show your calculations for Trial 1 in the space provided and then record your results in Table 2 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


