Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help me guys ahhhh Electrochemistry 4022143-3 Training questions (2) 1. The cell notation of a standard galvanic (voltaic) cell containing an unknown metal electrode X
help me guys ahhhh 
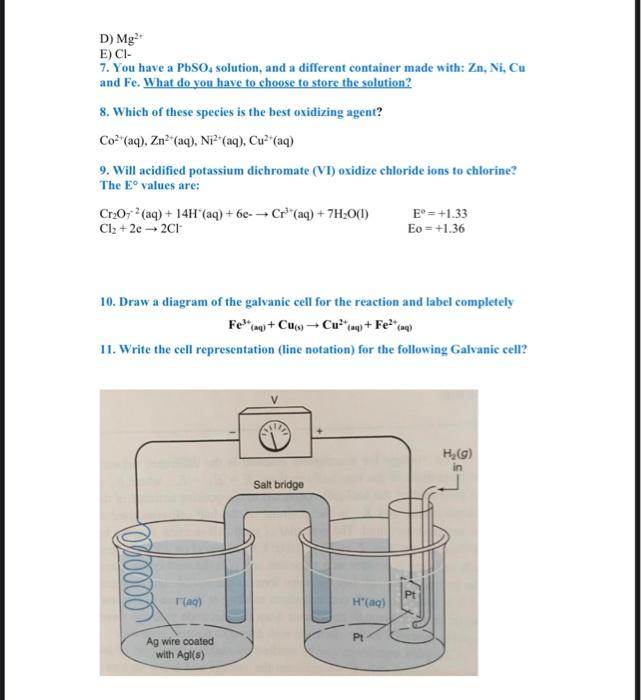
Electrochemistry 4022143-3 Training questions (2) 1. The cell notation of a standard galvanic (voltaic) cell containing an unknown metal electrode X is shown below. X(s)X3(1mol1m3)Pb2(1mol2m3)Pb(s) a) Name the component of the cell represented by the double vertical lines (I) in the above cell notation and explain its function. b) Identify the oxidizing agent in the above cell. c) The initial reading on a voltmeter connected across the electrodes of the above cell is 1.53V. Identify metal X by calculating the standard reduction potential of the unknown metal X. d) Write down the balanced equation for the net (overall) reaction taking place in this cell. Omit the spectator ions. e) How will the initial voltmeter reading be affected if the concentration of the electrolyte in the X(s)X3+(aq) half-cell is increased? Write down only INCREASES, DECREASES or REMAINS THE SAME 2. The cell contains two electrodes separated by a salt bridge, the first is Mg electrode immersed in its ions and another is Ni electrode immersed in its ions. Write the line notation and draw a diagram of the galvanic cell for the reaction and label completely 3. A standard hydrogen clectrode is connected to an Ag/Ag half-cell. (a) In what direction do the electrons flow in the extermal circuit? (b) A salt bridge of NH1NO3(aq) connects the two half-cells. What happens to the ions in this solution as the cell operates? (c) Does the concentration of H(aq) increase or decrease in the hydrogen electrode? (d) In this galvanic cell, what should be the sign of the charge marked on the hydrogen electrode? (e) Which electrode is the cathode? 4. How many electrons are transferred in the following reaction? 2Cr2O72++14H++6Cl22Cr3++3Cl2+7H2O 5. Which metal, Al, or Ni, could reduce Zn2+ to Zn(s) if placed in a Zn2+(aq) solution? Ni2++2eNi=0.23VZn2++2eZnE=0.76VAl3+3eAlE=1.66V3 6. Which of the following is the best reducing agent? A) Cl2 B) H2 C) Mg D) Mg2+ E) Cl - 7. You have a PbSO4 solution, and a different container made with: Zn,Ni,Cu and Fe. What do you have to choose to store the solution? 8. Which of these species is the best oxidizing agent? Co2+(aq),Zn2+(aq),Ni2+(aq),Cu2+(aq) 9. Will acidified potassium dichromate (VI) oxidize chloride ions to chlorine? The E values are: Cr2O72(aq)+14H+(aq)+6eCr3+(aq)+7H2O(I)Cl2+2e2ClE0=+1.33Eo=+1.36 10. Draw a diagram of the galvanic cell for the reaction and label completely Fe3+(aq)+Cu(s)Cu2+(aq)+Fe2+(aq) 11. Write the cell representation (line notation) for the following Galvanic cell 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


