Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help with all of these please!! I. At the endpoint of a titration, the color of the solution should be pinkbrightpinkfaintpinkcolorless II. We will calculate
help with all of these please!! 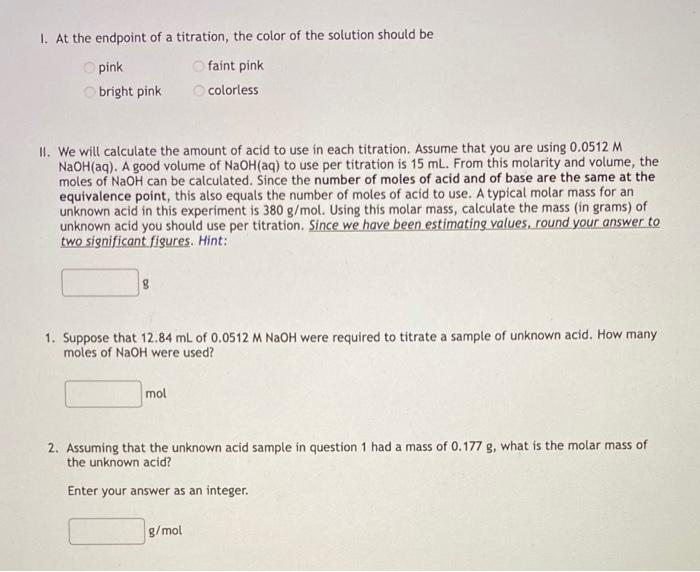
I. At the endpoint of a titration, the color of the solution should be pinkbrightpinkfaintpinkcolorless II. We will calculate the amount of acid to use in each titration. Assume that you are using 0.0512M NaOH(aq). A good volume of NaOH(aq) to use per titration is 15mL. From this molarity and volume, the moles of NaOH can be calculated. Since the number of moles of acid and of base are the same at the equivalence point, this also equals the number of moles of acid to use. A typical molar mass for an unknown acid in this experiment is 380g/mol. Using this molar mass, calculate the mass (in grams) of unknown acid you should use per titration. Since we have been estimating values, round your answer to two significant figures. Hint: 1. Suppose that 12.84mL of 0.0512MNaOH were required to titrate a sample of unknown acid. How many moles of NaOH were used? 2. Assuming that the unknown acid sample in question 1 had a mass of 0.177g, what is the molar mass of the unknown acid? Enter your answer as an integer. g/mol 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


