Question
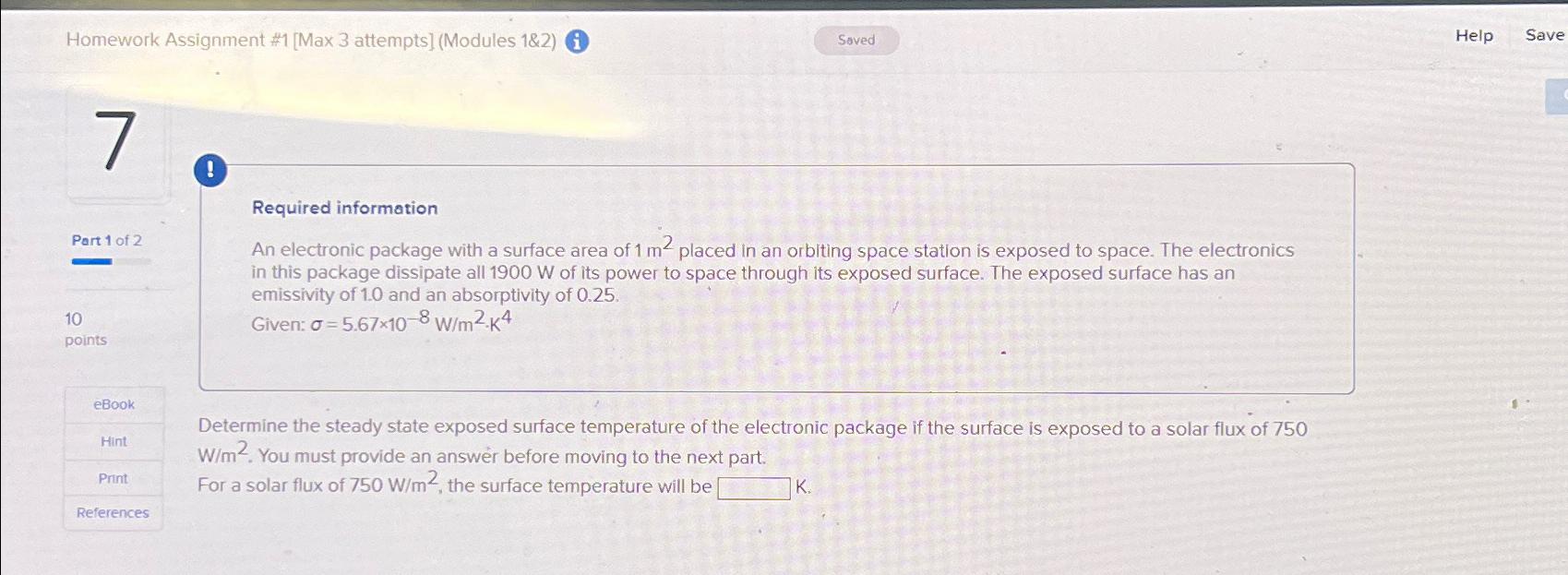
Homework Assignment #1 [Max 3 attempts] (Modules 1&2) (i) Help Save ! Required information Part 1 of 2 10 points eBook Hint Print References An
Homework Assignment
#1[Max 3 attempts] (Modules 1&2) (i)\ Help\ Save\ !\ Required information\ Part 1 of 2\ 10\ points\ eBook\ Hint\ Print\ References\ An electronic package with a surface area of
1m^(2)placed in an orbiting space station is exposed to space. The electronics in this package dissipate all
1900Wof its power to space through its exposed surface. The exposed surface has an emissivity of 1.0 and an absorptivity of 0.25 .\ Given:
\\\\sigma =5.67\\\\times 10^(-8)(W)/(m^(2))*K^(4)\ Determine the steady state exposed surface temperature of the electronic package if the surface is exposed to a solar flux of 750
(W)/(m^(2)). You must provide an answer before moving to the next part.\ For a solar flux of
750(W)/(m^(2)), the surface temperature will be\ K.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


