Answered step by step
Verified Expert Solution
Question
1 Approved Answer
how to do this excell? 2.00 (50 pts) Revisit problem 2 of HW 5. Add the R2 to the non-linear regression done in HW5 and
how to do this excell? 2.00
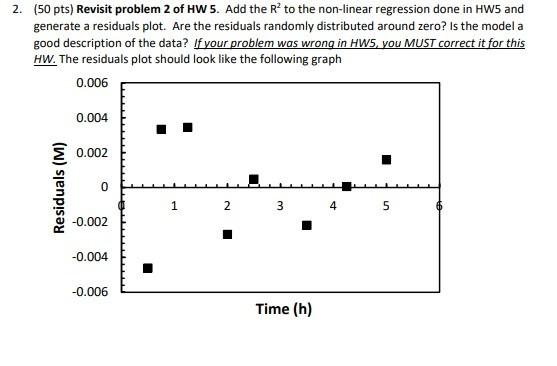
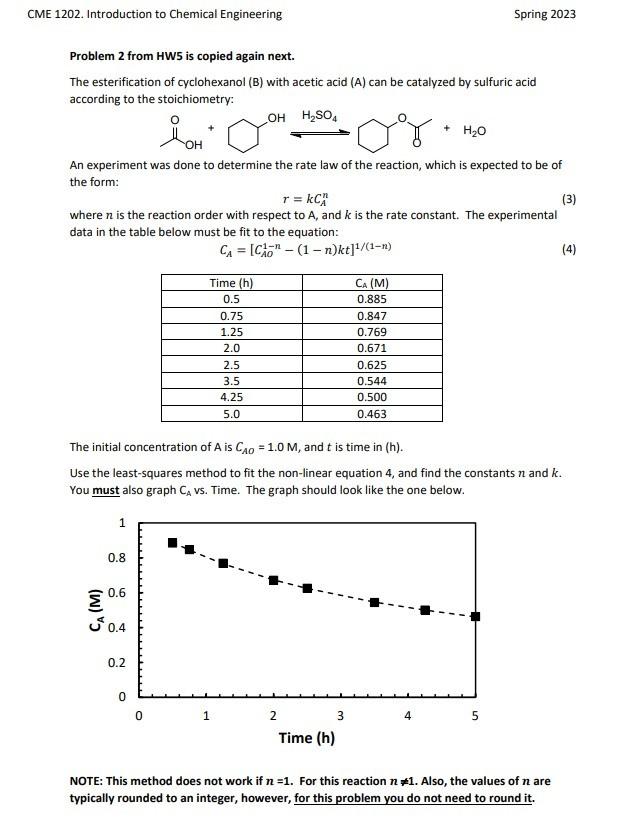
(50 pts) Revisit problem 2 of HW 5. Add the R2 to the non-linear regression done in HW5 and generate a residuals plot. Are the residuals randomly distributed around zero? Is the model a good description of the data? If your problem was wrong in HW5, you MUST correct it for this HW. The residuals plot should look like the following graph Problem 2 from HW5 is copied again next. The esterification of cyclohexanol (B) with acetic acid (A) can be catalyzed by sulfuric acid according to the stoichiometrv: An experiment was done to determine the rate law of the reaction, which is expected to be of the form: r=kCAn where n is the reaction order with respect to A, and k is the rate constant. The experimental data in the table below must be fit to the equation: CA=[CAO1n(1n)kt]1/(1n) The initial concentration of A is CAO=1.0M, and t is time in (h). Use the least-squares method to fit the non-linear equation 4 , and find the constants n and k. You must also graph CA vs. Time. The graph should look like the one below. NOTE: This method does not work if n=1. For this reaction n=1. Also, the values of n are typically rounded to an integer, however, for this problem you do not need to round it. (50 pts) Revisit problem 2 of HW 5. Add the R2 to the non-linear regression done in HW5 and generate a residuals plot. Are the residuals randomly distributed around zero? Is the model a good description of the data? If your problem was wrong in HW5, you MUST correct it for this HW. The residuals plot should look like the following graph Problem 2 from HW5 is copied again next. The esterification of cyclohexanol (B) with acetic acid (A) can be catalyzed by sulfuric acid according to the stoichiometrv: An experiment was done to determine the rate law of the reaction, which is expected to be of the form: r=kCAn where n is the reaction order with respect to A, and k is the rate constant. The experimental data in the table below must be fit to the equation: CA=[CAO1n(1n)kt]1/(1n) The initial concentration of A is CAO=1.0M, and t is time in (h). Use the least-squares method to fit the non-linear equation 4 , and find the constants n and k. You must also graph CA vs. Time. The graph should look like the one below. NOTE: This method does not work if n=1. For this reaction n=1. Also, the values of n are typically rounded to an integer, however, for this problem you do not need to round it
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


