Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I am theoretically designing a distillation system in which acetonitrile and acetic acid are separated. The substance being separated is acetonitrile. I do my theoretical
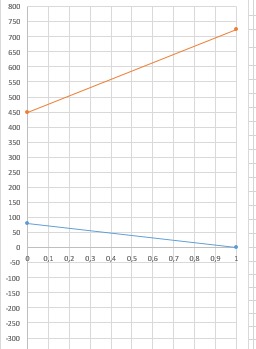
I am theoretically designing a distillation system in which acetonitrile and acetic acid are separated. The substance being separated is acetonitrile. I do my theoretical tray calculations with McCabe Thiele and Ponchon Savarit methods. In the vapor phase enthalpies used in the PonchonSavarit method, I found the slope of the vapor line to be positive because the enthalpy of pure acetonitrile in the vapor phase is higher than acetid acid. In general, the vapor enthalpy of the separated material is lower.
How can I interpret this? Can I do a tray calculation according to this graph? Since it is a completely theoretical system, you can explain all the disadvantages of the system efficiency value, column height, etc.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


