Question
i. In step (a), the hydrazone 8 reacts with 2 equivalents of butyl lithium (BuLi) to generate the anion 14. Propose a mechanism for this
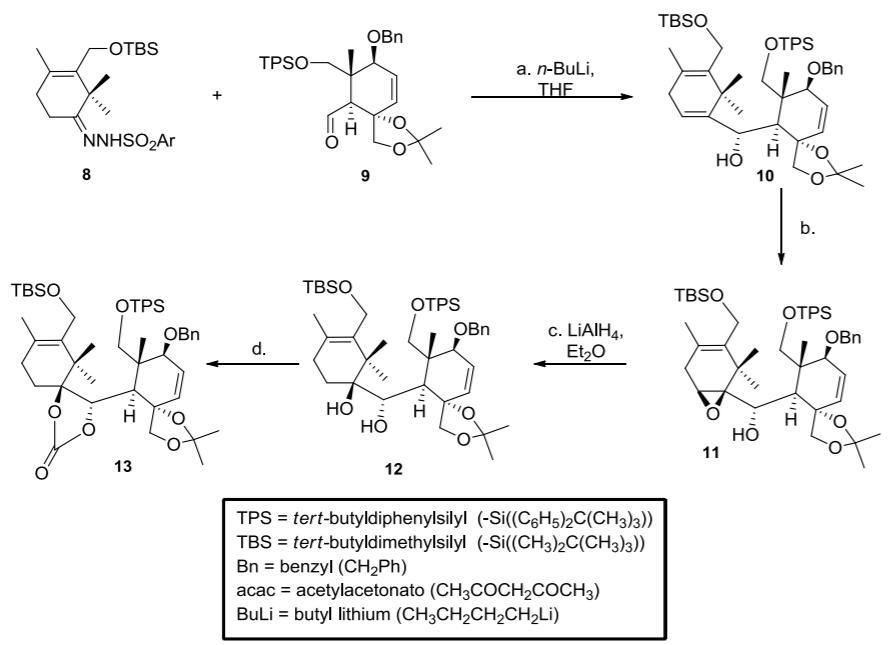
i. In step (a), the hydrazone 8 reacts with 2 equivalents of butyl lithium (BuLi) to generate the anion 14. Propose a mechanism for this conversion. (4 marks)

ii. Identify and name the type(s) of selectivity occurring in step (b). (2 marks)
iii. Step (d) is accomplished using carbonyl diimidizole 15. Outline a mechanism for this transformation. (3 marks)

iv. Draw compound 13 in your answer book and circle all the protecting groups (1 mark)
v. What reagent(s) would you choose to selectively remove just the silyl ethers from 13? (1 mark)
vi. Using your answers to parts (v) and (vi) above, explain what is meant by an orthogonal protecting group strategy. (1 mark)
TBSO OBn OTPS OBn OTBS TPSO- a. n-BuLi, THE NNHSO2A. H 10 8 TBSO TBSO TBSO OTPS OTPS PS c. LIAIH4, Et,0 OBn OBn OBn d. H. to to 11 13 12 TPS = tert-butyldiphenylsilyl (-Si((C6H5)2C(CH3)3)) TBS = tert-butyldimethylsilyl (-Si((CH3)2C(CH3)3)) Bn = benzyl (CH2PH) acac = acetylacetonato (CH3COCH2COCH3) BuLi = butyl lithium (CH3CH2CH2CH2LI) b. .....
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


