Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I know how to find the xsolvent for the pvap. But I don't understand why we calculated 15.6/0.84+15.6. I thought it was 0.84/15.6+0.84. Can someone
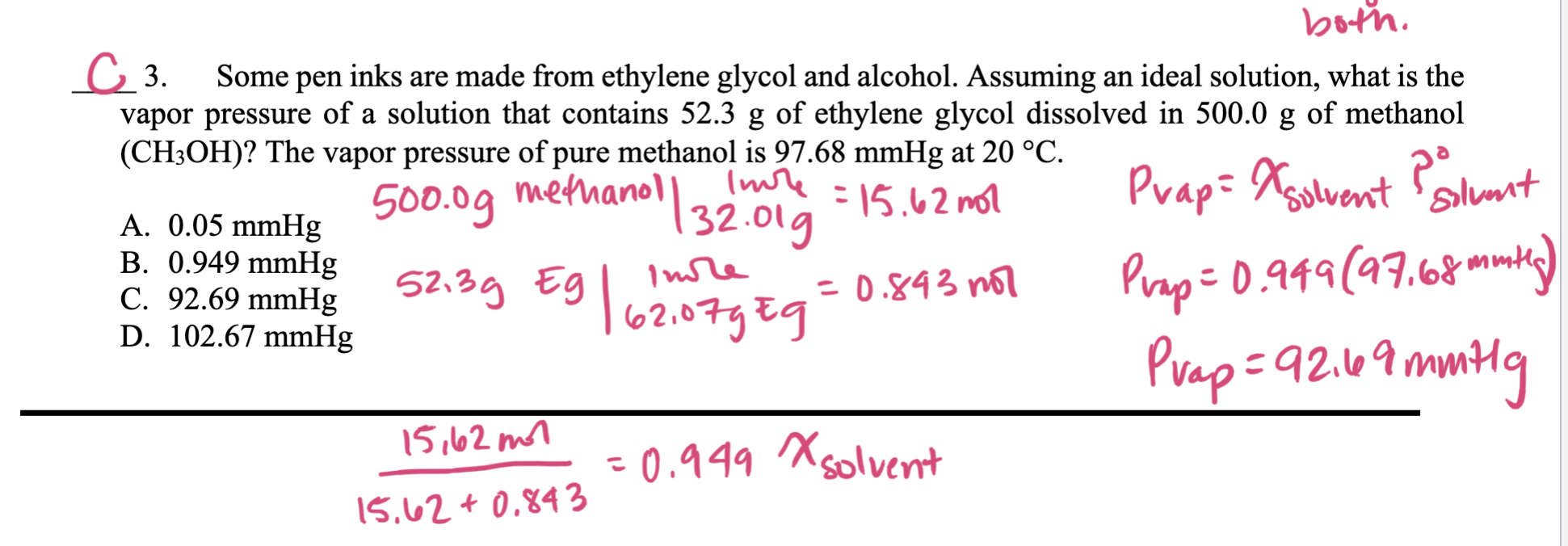
I know how to find the xsolvent for the pvap. But I don't understand why we calculated 15.6/0.84+15.6. I thought it was 0.84/15.6+0.84. Can someone please explain? Am I just getting the solvent and the solute mixed up? This is not a question about how to solve it, but rather I am just asking why my professor used the formula for xsolute when we should be using xsolvent.
C 3. Some pen inks are made from ethylene glycol and alcohol. Assuming an ideal solution, what is the vapor pressure of a solution that contains 52.3g of ethylene glycol dissolved in 500.0g of methanol (CH3OH) ? The vapor pressure of pure methanol is 97.68mmHg at 20C. A. 0.05mmHg500.0g methanol| 32.01g=15.62molPvap=XsolventPsolvent0 B.0.949mmHgC.92.69mmHgD.102.67mmHg52.3gEEg62.07gging=0.843molPrap=0.949(97.68mmtl) Pvap=92.69mm+1g 15.62+0.84315,62mn=0.949xsolventStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


