Answered step by step
Verified Expert Solution
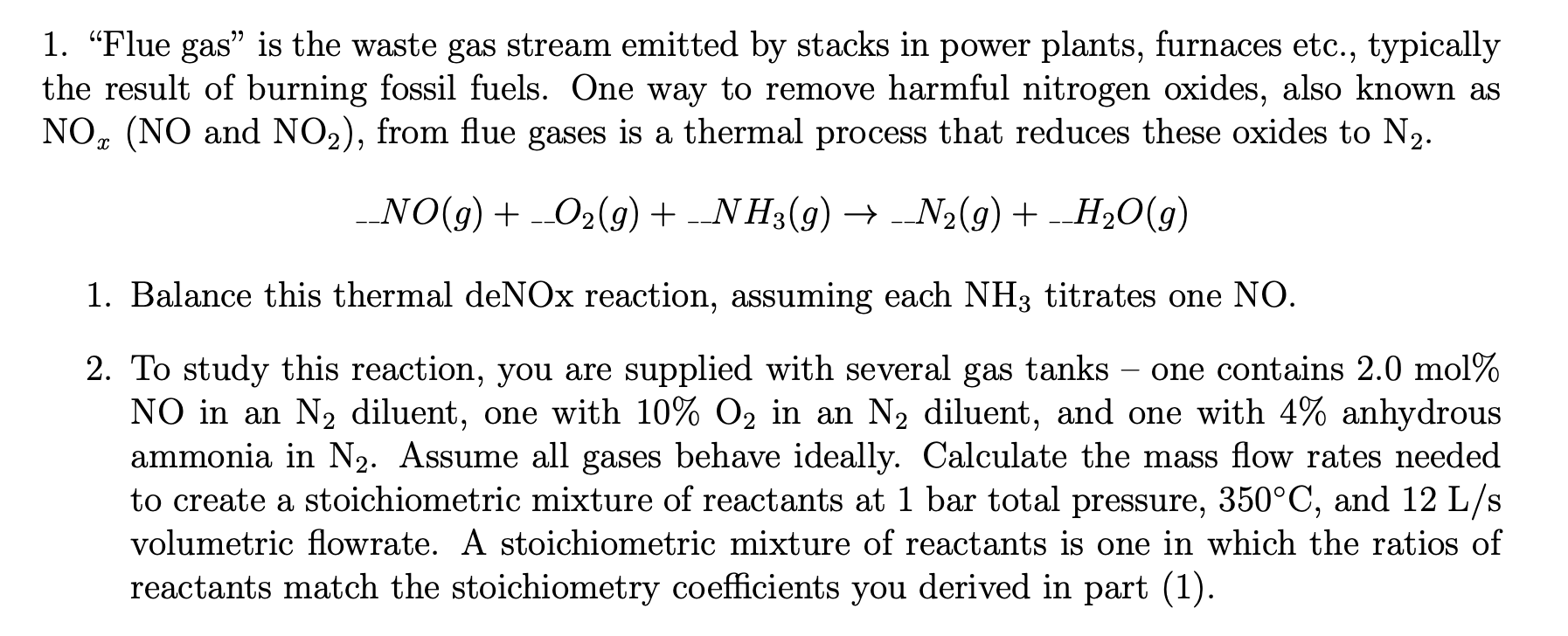
Question
1 Approved Answer
I need help on questions 2 , I have question 1 , but I would still like to double check I have the correct answer.
I need help on questions I have question
but I would still like to double check I have the correct answer. I will leave an upvote. Blance this thermal deNOx reaction, assuming each titrates one
To study this reaction, you are supplied with several gas tanks one contains mol
in an diluent, one with in an diluent, and one with anhydrous
ammonia in Assume all gases behave ideally. Calculate the mass flow rates needed
to create a stoichiometric mixture of reactants at bar total pressure, and
volumetric flowrate. A stoichiometric mixture of reactants is one in which the ratios of
reactants match the stoichiometry coefficients you derived in part I need help on questions I have question but I would still like to double check I have the correct answer. I will leave an upvoteI need help on questions I have question but I would still like to double check I have the correct answer. I will leave an upvoteI need help on questions I have question but I would still like to double check I have the correct answer. I will leave an upvoteI need help on questions I have question but I would still like to double check I have the correct answer. I will leave an upvote
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


