Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help with problem 9.19 use 10 bar as pressure. Table 9.8 Boiling Temperature Data for the System A-B-C in Exercise 9.18 Table 9.10
I need help with problem 9.19 use 10 bar as pressure.
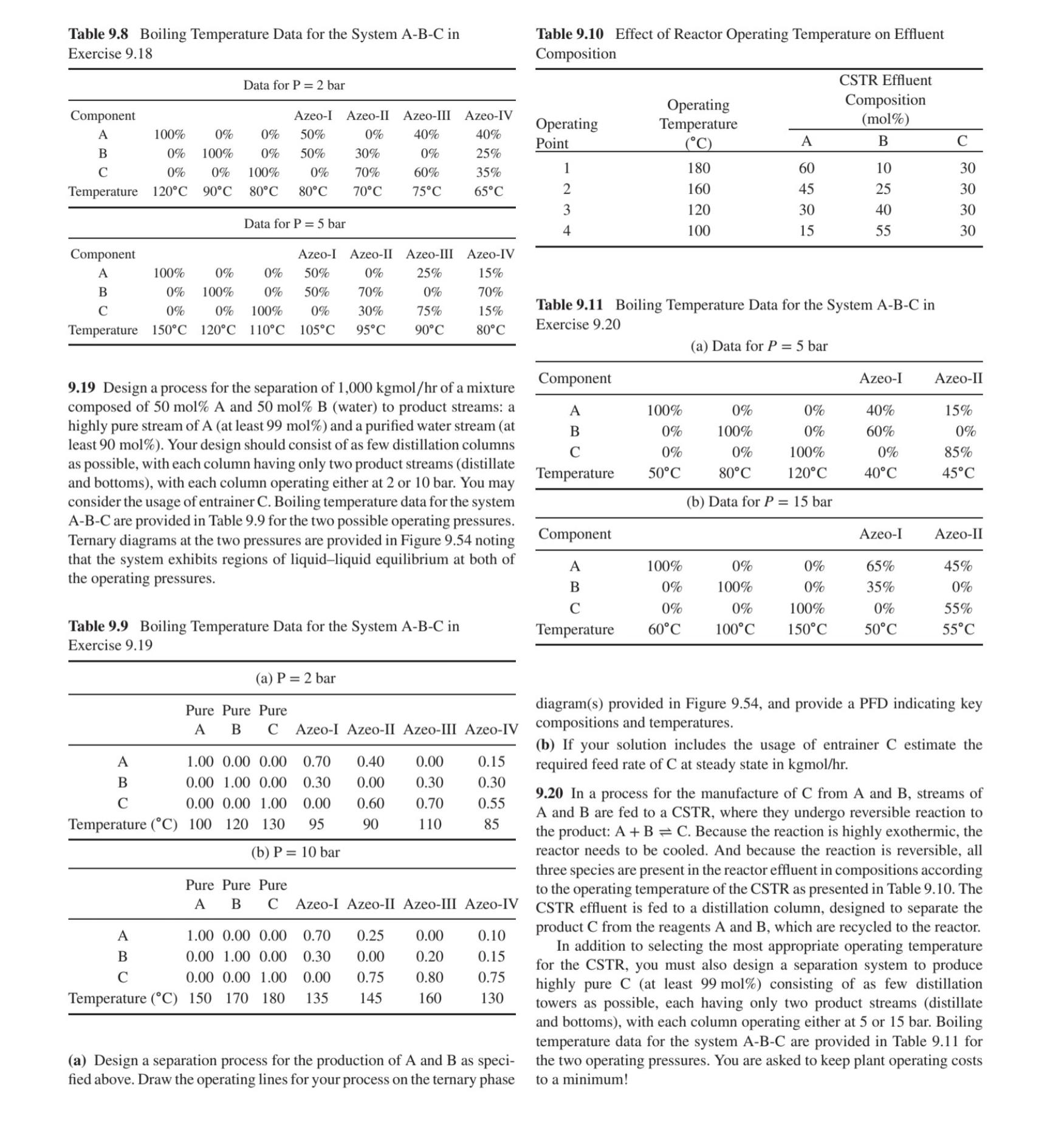
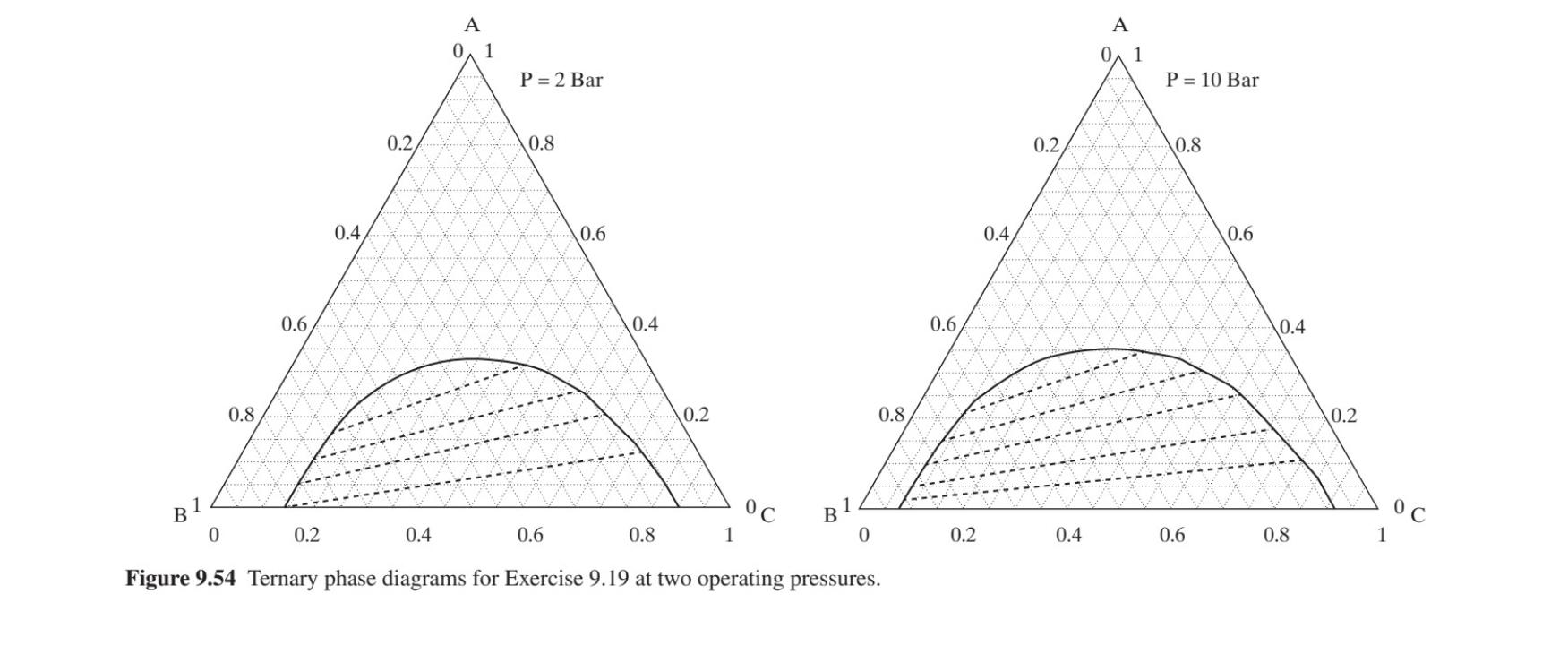
Table 9.8 Boiling Temperature Data for the System A-B-C in Exercise 9.18 Table 9.10 Effect of Reactor Operating Temperature on Effluent Composition Data for P = 2 bar CSTR Effluent Component Azeo-I Azeo-II Azeo-III Azeo-IV Operating Operating Temperature Composition (mol%) A 100% 0% 0% 50% 0% 40% 40% Point (C) A B C B C 0% 100% 0% 50% 30% 0% 25% 0% 0% 100% 1 180 60 0% 70% 60% 35% 10 30 Temperature 120C 90C 80C 80C 70C 75C 65C 2 160 45 25 30 3 120 30 40 30 Data for P 5 bar 4 100 15 55 30 Component A B 100% 0% 100% 0% Azeo-I Azeo-II Azeo-III Azeo-IV 0% 50% 0% 0% 50% 70% 0% 0% 100% 0% 30% Temperature 150C 120C 110C 105C 25% 0% 75% 15% 70% 15% 95C 90C 80C Table 9.11 Boiling Temperature Data for the System A-B-C in Exercise 9.20 (a) Data for P = 5 bar Component Azeo-I Azeo-II 9.19 Design a process for the separation of 1,000 kgmol/hr of a mixture composed of 50 mol% A and 50 mol% B (water) to product streams: a highly pure stream of A (at least 99 mol%) and a purified water stream (at least 90 mol%). Your design should consist of as few distillation columns as possible, with each column having only two product streams (distillate and bottoms), with each column operating either at 2 or 10 bar. You may consider the usage of entrainer C. Boiling temperature data for the system A-B-C are provided in Table 9.9 for the two possible operating pressures. Ternary diagrams at the two pressures are provided in Figure 9.54 noting that the system exhibits regions of liquid-liquid equilibrium at both of the operating pressures. A 100% 0% 0% 40% 15% B 0% 100% 0% 60% 0% C 0% 0% 100% 0% 85% Temperature 50C 80C 120C 40C 45C (b) Data for P = 15 bar Component Azeo-I Azeo-II A 100% 0% 0% 65% 45% B 0% 100% 0% 35% 0% C 0% 0% 100% 0% 55% Table 9.9 Boiling Temperature Data for the System A-B-C in Exercise 9.19 Temperature 60C 100C 150C 50C 55C (a) P = 2 bar Pure Pure Pure A A 1.00 0.00 0.00 0.70 0.40 0.00 0.15 B 0.00 1.00 0.00 0.30 0.00 0.30 C 0.00 0.00 1.00 Temperature (C) 100 120 130 95 0.00 0.60 0.70 0.30 0.55 90 110 85 (b) P= 10 bar Pure Pure Pure A B C A 1.00 0.00 0.00 0.70 0.25 0.00 0.10 B 0.00 1.00 0.00 0.30 0.00 0.20 C 0.00 0.00 1.00 0.00 0.75 0.80 Temperature (C) 150 170 180 135 145 160 0.15 0.75 130 diagram(s) provided in Figure 9.54, and provide a PFD indicating key B C Azeo-I Azeo-II Azeo-III Azeo-IV compositions and temperatures. (b) If your solution includes the usage of entrainer C estimate the required feed rate of C at steady state in kgmol/hr. 9.20 In a process for the manufacture of C from A and B, streams of A and B are fed to a CSTR, where they undergo reversible reaction to the product: A+B = C. Because the reaction is highly exothermic, the reactor needs to be cooled. And because the reaction is reversible, all three species are present in the reactor effluent in compositions according to the operating temperature of the CSTR as presented in Table 9.10. The Azeo-I Azeo-II Azeo-III Azeo-IV CSTR effluent is fed to a distillation column, designed to separate the (a) Design a separation process for the production of A and B as speci- fied above. Draw the operating lines for your process on the ternary phase product C from the reagents A and B, which are recycled to the reactor. In addition to selecting the most appropriate operating temperature for the CSTR, you must also design a separation system to produce highly pure C (at least 99 mol%) consisting of as few distillation towers as possible, each having only two product streams (distillate and bottoms), with each column operating either at 5 or 15 bar. Boiling temperature data for the system A-B-C are provided in Table 9.11 for the two operating pressures. You are asked to keep plant operating costs to a minimum!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


