Answered step by step
Verified Expert Solution
Question
1 Approved Answer
If the following reactions take place in a well mixed batch reactor with the assumptions of constant volume and elementary first order reactions, what are
If the following reactions take place in a well mixed batch reactor with the assumptions of constant volume and elementary first order reactions, what are the material balances for each species? Since H2O is the solvent and it is in excess, it's concentration does not affect the reaction and can be neglected.
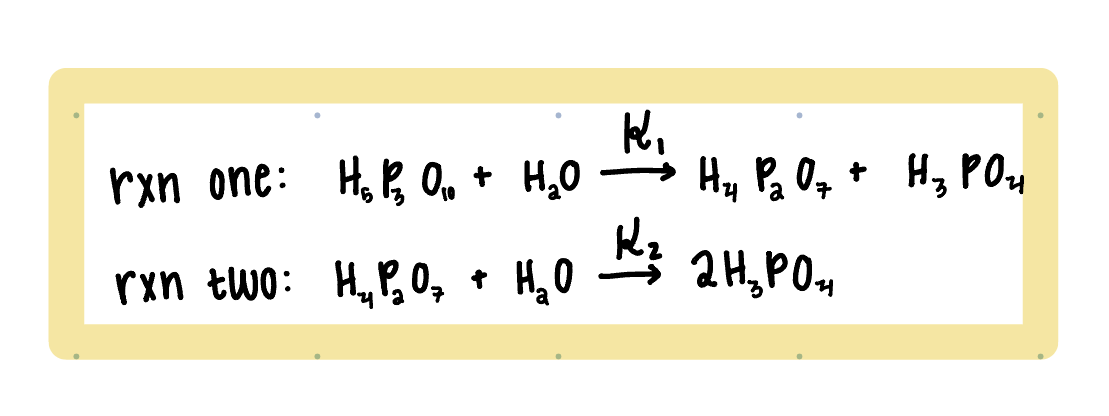
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


