Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I'm really confused on how to find concentration with only 2 volumes, %t, and absorbance. Part 1 of the question (first picture) regards the red
I'm really confused on how to find concentration with only 2 volumes, %t, and absorbance. Part 1 of the question (first picture) regards the red dye and the data I gathered is in the second picture. Part 2 of the questions (third picture) regards the green dye and the data I gathered is in the fourth picture. Thanks so much! 
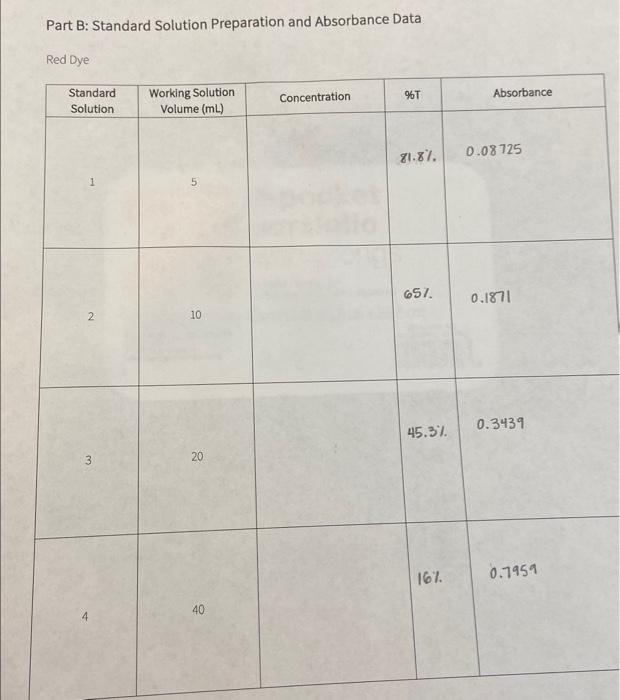

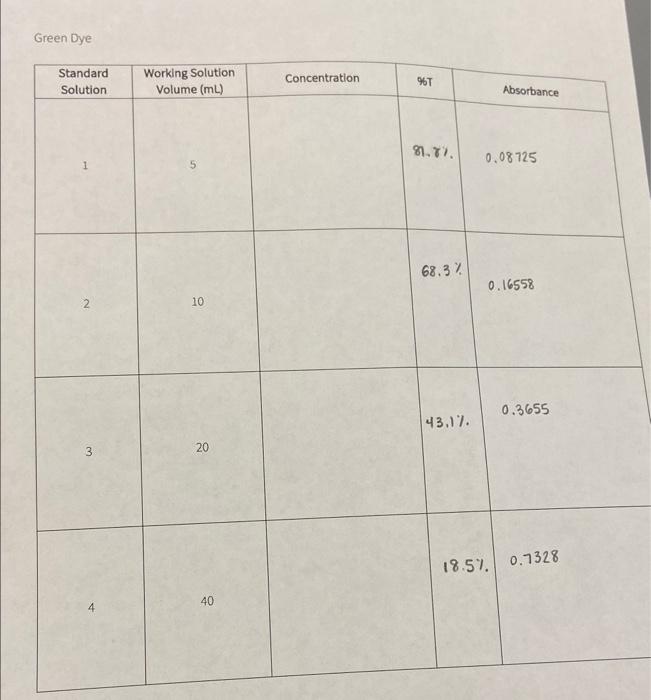
Calculate the concentration of working solution of red dye. Note: Use M1V1=M2V2.M1 is the concentration of standard solution, V1 is the volume of standard solution, V2 is the volume of final diluted solution, and M2 is the concentration of working solution. Record the answer to 3 decimal places. Concentration of Standard solution Volume of standard solution used Total volume of working solution after dilution Cancentration of workins solution Part B: Standard Solution Preparation and Absorbance Data Calculate the concentration of working solution of green dye. Note: Use M1V1=M2V2.M1 is the concentration of standard solution, V1 is the volume of standard solution, V2 is the volume of final diluted solution, and M2 is the concentration of working solution. Record the answer to 3 decimal places. Concentration of Standard solution Volume of standard solution used Total volume of working solution after dilution Concentration of working solution Green Dye 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


