Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In a countercurrent extractor, NaOH is extracted from the CaCO3NaOH mixture with pure water. The feed mixture contains 1000kg/hCaCO3 (inert), 100kg/hNaOH and 50kg/hH2O. The extract
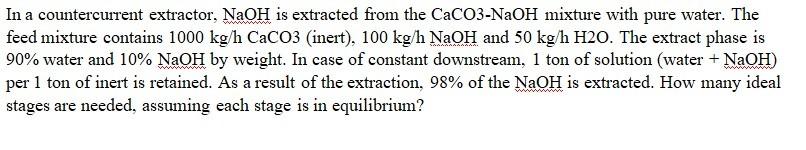
In a countercurrent extractor, NaOH is extracted from the CaCO3NaOH mixture with pure water. The feed mixture contains 1000kg/hCaCO3 (inert), 100kg/hNaOH and 50kg/hH2O. The extract phase is 90% water and 10%NaOH by weight. In case of constant downstream, 1 ton of solution (water +NaOH ) per 1 ton of inert is retained. As a result of the extraction, 98% of the NaOH is extracted. How many ideal stages are needed, assuming each stage is in equilibrium
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


