Answered step by step
Verified Expert Solution
Question
1 Approved Answer
IN A PFR REACTOR IT IS PLANNED TO CARRY OUT THE FOLLOWING ELEMENTARY REVERSIBLE CHEMICAL REACTION IN GASEOUS PHASE: THE FEEDING SCHEME AND OTHER DATA
IN A PFR REACTOR IT IS PLANNED TO CARRY OUT THE FOLLOWING ELEMENTARY REVERSIBLE CHEMICAL REACTION IN GASEOUS PHASE:
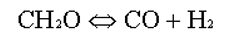 THE FEEDING SCHEME AND OTHER DATA FOR THE REACTOR ARE SHOWN IN THE FOLLOWING FIGURE:
THE FEEDING SCHEME AND OTHER DATA FOR THE REACTOR ARE SHOWN IN THE FOLLOWING FIGURE:
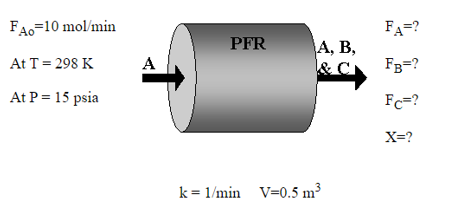 A) DETERMINE THE EQUILIBRIUM CONVERSION IF AT 298K THE EQUILIBRIUM CONSTANT KC=21980 B) DETERMINE THE CURRENT DAILY PRODUCTION OF CO AND H2. C) DETERMINE THE ACHIEVABLE CONVERSION IF THE REACTOR WERE REPLACED BY A 750-LITRE PFR REACTOR.
A) DETERMINE THE EQUILIBRIUM CONVERSION IF AT 298K THE EQUILIBRIUM CONSTANT KC=21980 B) DETERMINE THE CURRENT DAILY PRODUCTION OF CO AND H2. C) DETERMINE THE ACHIEVABLE CONVERSION IF THE REACTOR WERE REPLACED BY A 750-LITRE PFR REACTOR.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


