Answered step by step
Verified Expert Solution
Question
1 Approved Answer
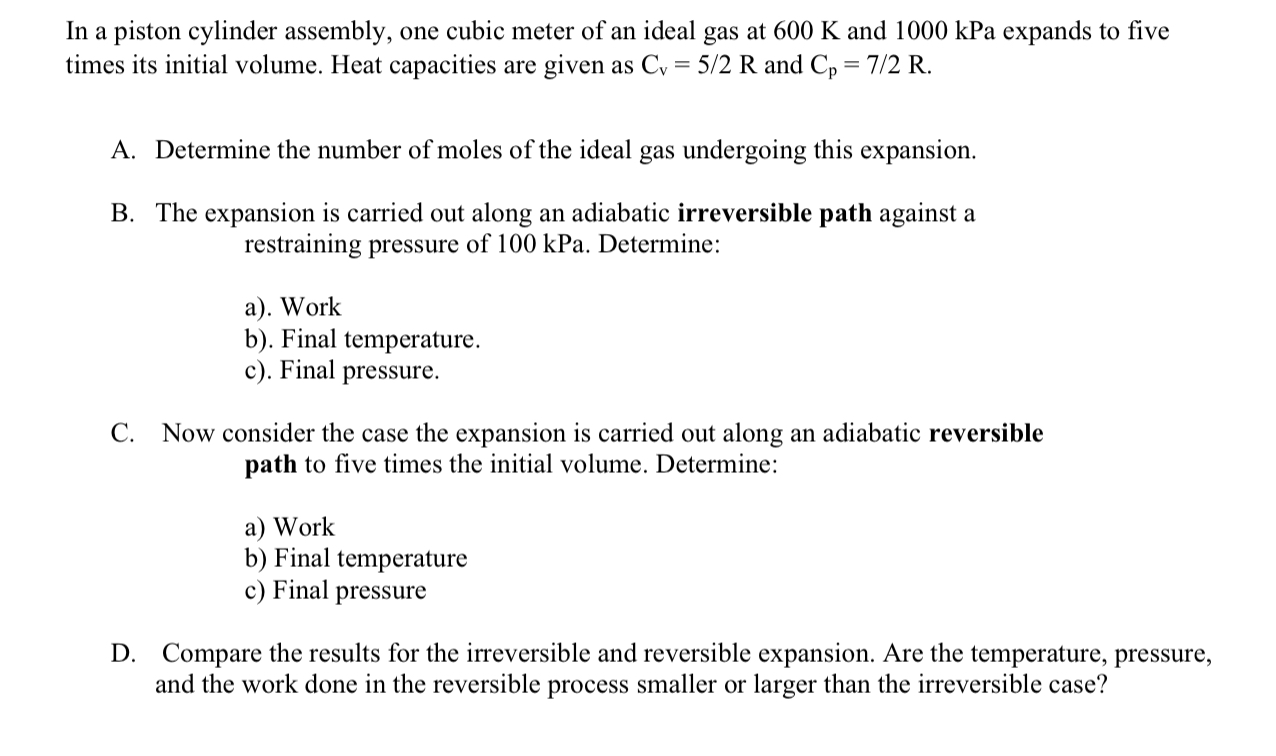
In a piston cylinder assembly, one cubic meter of an ideal gas at 6 0 0 K and 1 0 0 0 kPa expands to
In a piston cylinder assembly, one cubic meter of an ideal gas at and kPa expands to five
times its initial volume. Heat capacities are given as and
A Determine the number of moles of the ideal gas undergoing this expansion.
B The expansion is carried out along an adiabatic irreversible path against a
restraining pressure of kPa. Determine:
a Work
b Final temperature.
c Final pressure.
C Now consider the case the expansion is carried out along an adiabatic reversible
path to five times the initial volume. Determine:
a Work
b Final temperature
c Final pressure
D Compare the results for the irreversible and reversible expansion. Are the temperature, pressure,
and the work done in the reversible process smaller or larger than the irreversible case?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


