in need of help ASAP!!!! I have provided all of the data needed. Just need the part D ones answered. thank you!
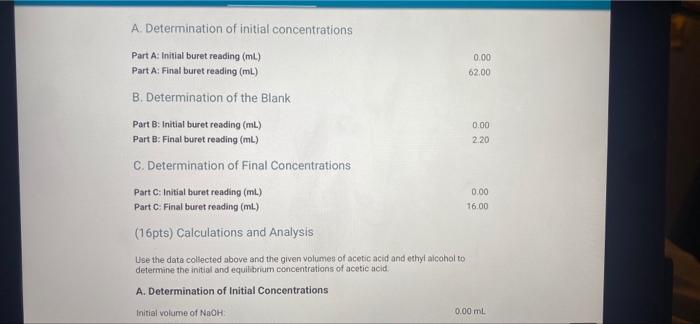
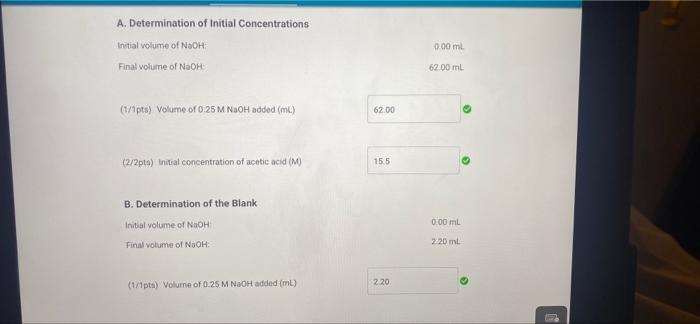
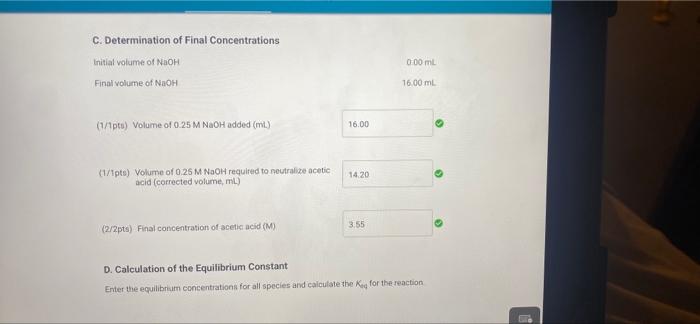
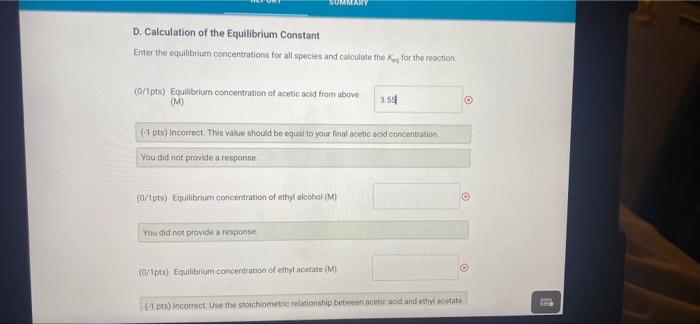
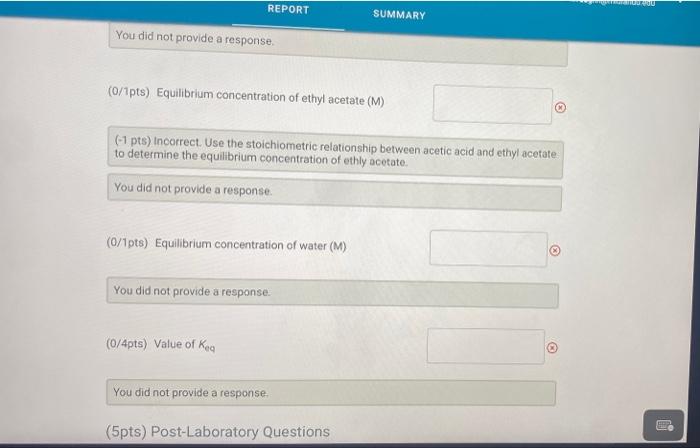
0.00 62.00 A. Determination of initial concentrations Part A: Initial buret reading (ml) Part A Final buret reading (mL) B. Determination of the Blank Part B: Initial buret reading (mL) Part 8: Final buret reading (ml) C. Determination of Final Concentrations Part CInitial buret reading (mi) Part C. Final buret reading (ml) 0.00 2.20 0.00 16.00 (16pts) Calculations and Analysis Use the data collected above and the given volumes of acetic acid and ethyl alcohol to determine the initial and equilibrium concentrations of acetic acid A. Determination of initial Concentrations Initial volume of NaOH: 0.00 ml A. Determination of Initial Concentrations Initial volume of NaOH 0.00 mL Final volume of NaOH 62.00 mL (7/1pts) Volume of 0.25 M NaOH added (m.) 62.00 (2/2pts) initial concentration of acetic acid (M) 15.5 B. Determination of the Blank Initial volume of NAOH 0.00 mL Final volume of NaOH: 220 ml 2.20 (1/1pts) Volume of 0.25 M NaOH added (mL) C. Determination of Final Concentrations Initial volume of NaOH Final volume of NICH 0.00 mL 16.00 ml (1/1pta) Volume of 0.25 M NaOH added (m.) 16.00 14.20 (1/1pts) Volume of 0.25 M NaOH required to neutralize acetic acid (corrected volume, ml) 3.55 (2/2pts) Final concentration of acetic acid (M) D. Calculation of the Equilibrium Constant Enter the equilibrium concentrations for all species and calculate the key for the reaction SUMMARY D. Calculation of the Equilibrium Constant Enter the equilibrium concentrations for all species and calculate the K, for the reaction (0/1pts) Equilibrium concentration of acetic acid from above (M) 3.5 (7 pt) Incorrect. Thin value should be equal to your final scetic acid concentration You did not provide a response (0/1pts) Equilibrium concentration of ethyl alcohol (M) You did not provide a response (0/1pts) Equilibrium concentration of ethyl acetate (M) 1 pts) incorrect Use the stoichiometric relationship between acetic acid and ethyl acetate URDU REPORT SUMMARY You did not provide a response (0/1pts) Equilibrium concentration of ethyl acetate (M) (-1 pts) incorrect. Use the stoichiometric relationship between acetic acid and ethyl acetate to determine the equilibrium concentration of ethly acetato. You did not provide a response. (0/1pts) Equilibrium concentration of water (M) You did not provide a response. (0/4pts) Value of Keo You did not provide a response. (5pts) Post-Laboratory Questions