Answered step by step
Verified Expert Solution
Question
1 Approved Answer
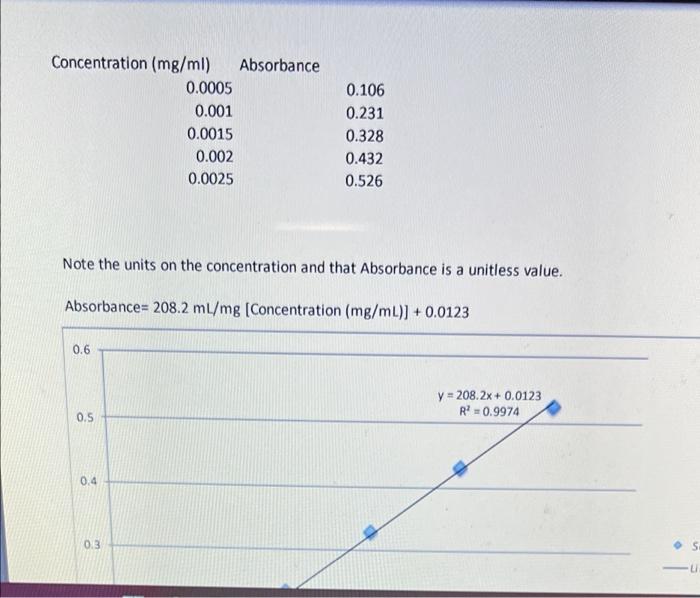
ineed help Thanks concetration of fhe iron is = 0.00480 Concentration (mg/ml) Absorbance 0.0005 0.001 0.0015 0.002 0.0025 0.106 0.231 0.328 0.432 0.526 Note the
 ineed help Thanks
ineed help Thanks 
concetration of fhe iron is = 0.00480
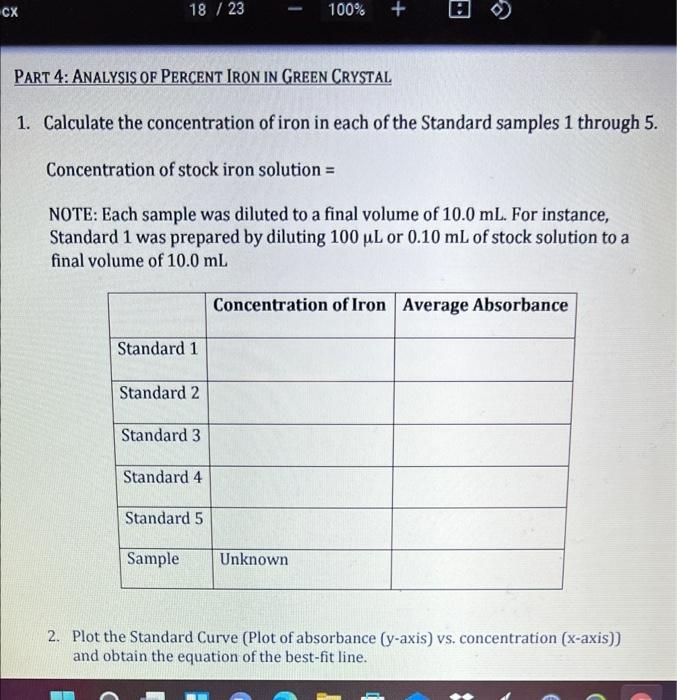
Concentration (mg/ml) Absorbance 0.0005 0.001 0.0015 0.002 0.0025 0.106 0.231 0.328 0.432 0.526 Note the units on the concentration and that Absorbance is a unitless value. Absorbance=208.2 ml/mg (Concentration (mg/mL)] +0.0123 0.6 y = 208.2x + 0.0123 R 0.9974 0.5 0.4 0.3 S u cx 18 / 23 100% + PART 4: ANALYSIS OF PERCENT IRON IN GREEN CRYSTAL 1. Calculate the concentration of iron in each of the Standard samples 1 through 5. Concentration of stock iron solution = NOTE: Each sample was diluted to a final volume of 10.0 mL. For instance, Standard 1 was prepared by diluting 100 uL or 0.10 mL of stock solution to a final volume of 10.0 mL Concentration of Iron Average Absorbance Standard 1 Standard 2 Standard 3 Standard 4 Standard 5 Sample Unknown 2. Plot the Standard Curve (Plot of absorbance (y-axis) vs. concentration (x-axis)) and obtain the equation of the best-fit line. E 3. Calculate the concentration of iron in the sample. 4. The above number is the concentration of the iron in the 10-ml volumetric flask sample prepared in Step 13 of the procedure. This sample was prepared by diluting 0.5 mL of the original 25.0 mL sample to a final volume of 10.0 mL. Using the concentration calculated in Step 3 above, calculate the concentration of iron in the 25.0 mL sample. 5. Calculate the grams of iron in the sample. NOTE: The volume of the sample is 25.0 mL and the concentration of iron is the number calculated in Step 4 above Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


