Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Last Question? ii question iii 2006 AP CHEMISTRY FREE-RESPONSE QUESTIONS (Form B) Your responses to the rest of the questions in this part of the
Last Question? ii
question iii 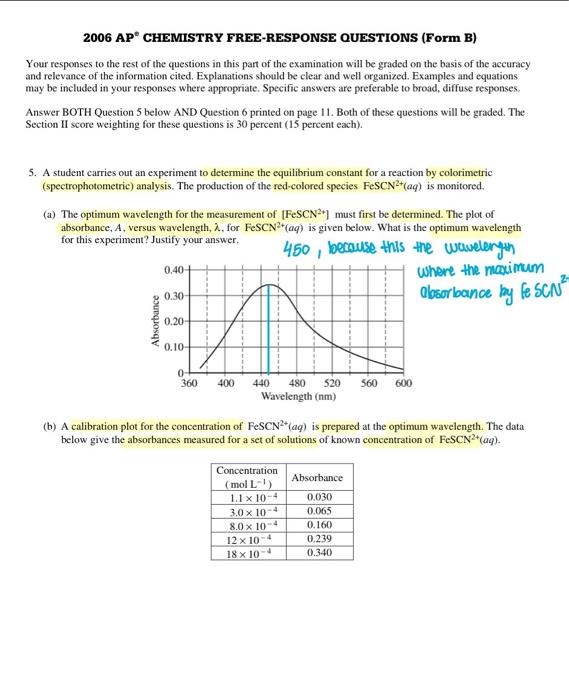
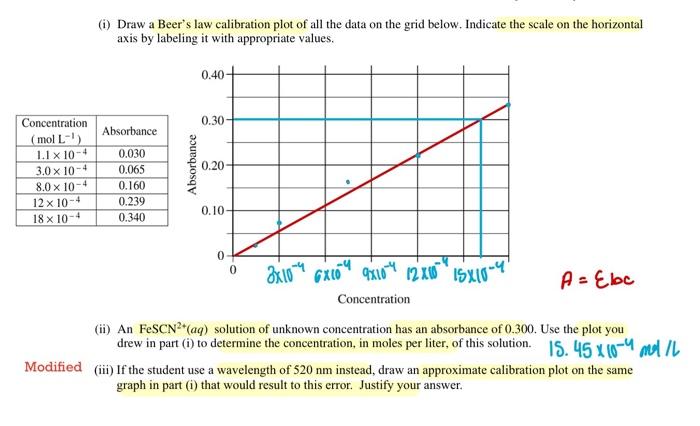
2006 AP CHEMISTRY FREE-RESPONSE QUESTIONS (Form B) Your responses to the rest of the questions in this part of the examination will be graded on the basis of the accuracy and relevance of the information cited. Explanations should be clear and well organized. Examples and equations may be included in your responses where appropriate. Specific answers are preferable to broad, diffuse responses. Answer BOTH Question 5 below AND Question 6 printed on page 11. Both of these questions will be graded. The Section II score weighting for these questions is 30 percent (15 percent each). 5. A student carries out an experiment to determine the equilibrium constant for a reaction by colorimetric (spectrophotometric) analysis. The production of the red-colored species FeSCN 2+(aq) is monitored. (a) The optimum wavelength for the measurement of [FeSCN 2] must first be determined. The plot of absorbance, A, versus wavelength, , for FeSCN2+(aq) is given below. What is the optimum wavelength for this experiment? Justify your answer. 450,10 erause this the wtawderith shere the naximunn losorbounce by fosce 2 (b) A calibration plot for the concentration of FeSCN2+(aq) is prepared at the optimum wavelength. The data below give the absorbances measured for a set of solutions of known concentration of FeSCN 2+(aq). (i) Draw a Beer's law calibration plot of all the data on the grid below. Indicate the scale on the horizontal axis by labeling it with appropriate values. A=bc (ii) An FeSCN2+(aq) solution of unknown concentration has an absorbance of 0.300. Use the plot you drew in part (i) to determine the concentration, in moles per liter, of this solution. 15.45104,01/5 ified (iii) If the student use a wavelength of 520nm instead, draw an approximate calibration plot on the same graph in part (i) that would result to this error. Justify your 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


